bmuodos Cthet Supposc 0.5160g of sample is added to 50.00 mL of 0.1000M HCl. The resulting solution required 39.50 mL Of 0.0980M NaOH to reach pH 3. What is the acid consuming capacity of the sample in moles of acid neutralized per gram of samplc? 5.
bmuodos Cthet Supposc 0.5160g of sample is added to 50.00 mL of 0.1000M HCl. The resulting solution required 39.50 mL Of 0.0980M NaOH to reach pH 3. What is the acid consuming capacity of the sample in moles of acid neutralized per gram of samplc? 5.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 40P
Related questions
Question
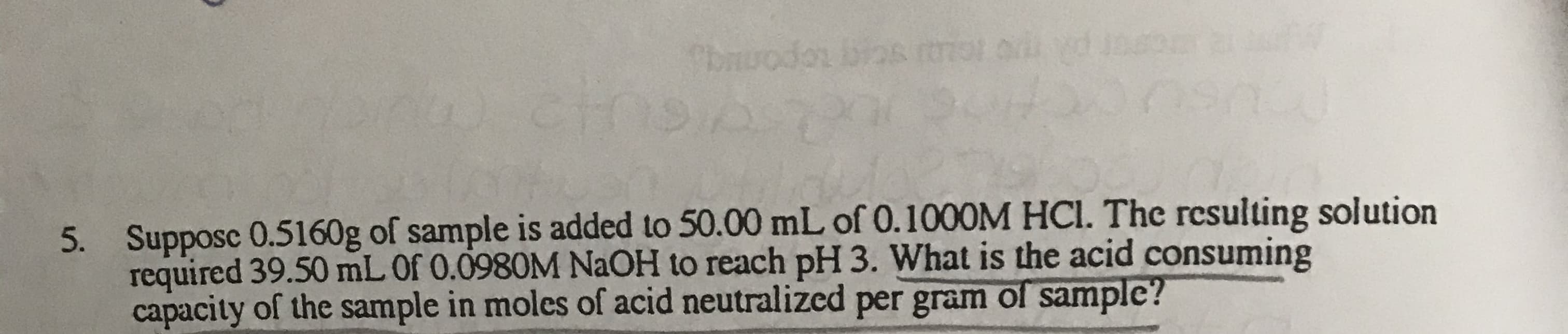
Transcribed Image Text:bmuodos
Cthet
Supposc 0.5160g of sample is added to 50.00 mL of 0.1000M HCl. The resulting solution
required 39.50 mL Of 0.0980M NaOH to reach pH 3. What is the acid consuming
capacity of the sample in moles of acid neutralized per gram of samplc?
5.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 8 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning