bOn average, would Earth be wa success -Fing x Course Home X + HSS Website openvellum.ecollege.com/course.html?courseld=155561588HeplD=6b9f9b2f... 2 01 0
bOn average, would Earth be wa success -Fing x Course Home X + HSS Website openvellum.ecollege.com/course.html?courseld=155561588HeplD=6b9f9b2f... 2 01 0
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter9: Gases
Section: Chapter Questions
Problem 55E: The density of a certain gaseous fluoride of phosphorus is 3.93 g/L at STP. Calculate the molar mass...
Related questions
Question
100%
Transcribed Image Text:bOn average, would Earth be wa
success -Fing x
Course Home
X
+
HSS Website
openvellum.ecollege.com/course.html?courseld=155561588HeplD=6b9f9b2f...
2
01 0
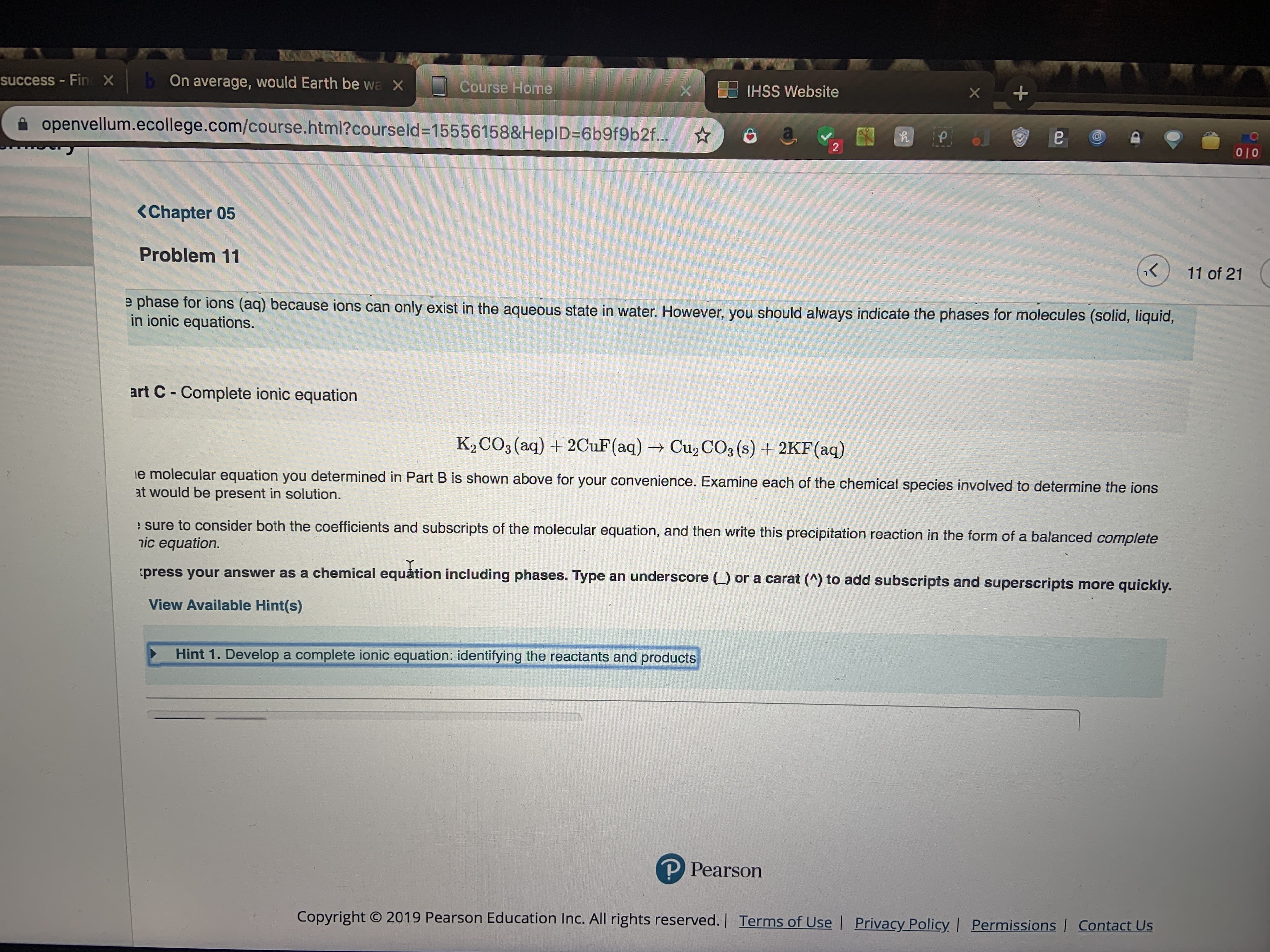
<Chapter 05
Problem 11
11 of 21
1
e phase for ions (aq) because ions can only exist in the aqueous state in water. However, you should always indicate the phases for molecules (solid, liquiad
in ionic equations.
art C Complete ionic equation
K2 CO3 (aq)2CuF (aq)Cu2 CO3 (s)+2KF(aq)
e molecular equation you determined in Part B is shown above for your convenience. Examine each of the chemical species involved to determine the ions
at would be present in solution.
sure to consider both the coefficients and subscripts of the molecular equation, and then write this precipitation reaction in the form of a balanced complete
nic equation.
press your answer as a chemical equation including phases. Type an underscore (_) or a carat (A) to add subscripts and superscripts more quickly.
View Available Hint(s)
Hint 1. Develop a complete ionic equation: identifying the reactants and products
P Pearson
Copyright O 2019 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions Contact Us
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 3 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning