(c) Suppose an alloy consists of a 75 wt?% Sn-25 wt% Pb. List the phases present in the alloy at 185 °C. ii. Determine the composition of the phases present in (i). Calculate the relative amount of each phase present at 183 °C in terms of the mass fraction.
(c) Suppose an alloy consists of a 75 wt?% Sn-25 wt% Pb. List the phases present in the alloy at 185 °C. ii. Determine the composition of the phases present in (i). Calculate the relative amount of each phase present at 183 °C in terms of the mass fraction.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter23: Potentiometry
Section: Chapter Questions
Problem 23.12QAP: What arc the advantages of microfabricated ISEs? Describe typical applications of this type of...
Related questions
Question
Please solve all question. I give thumb up
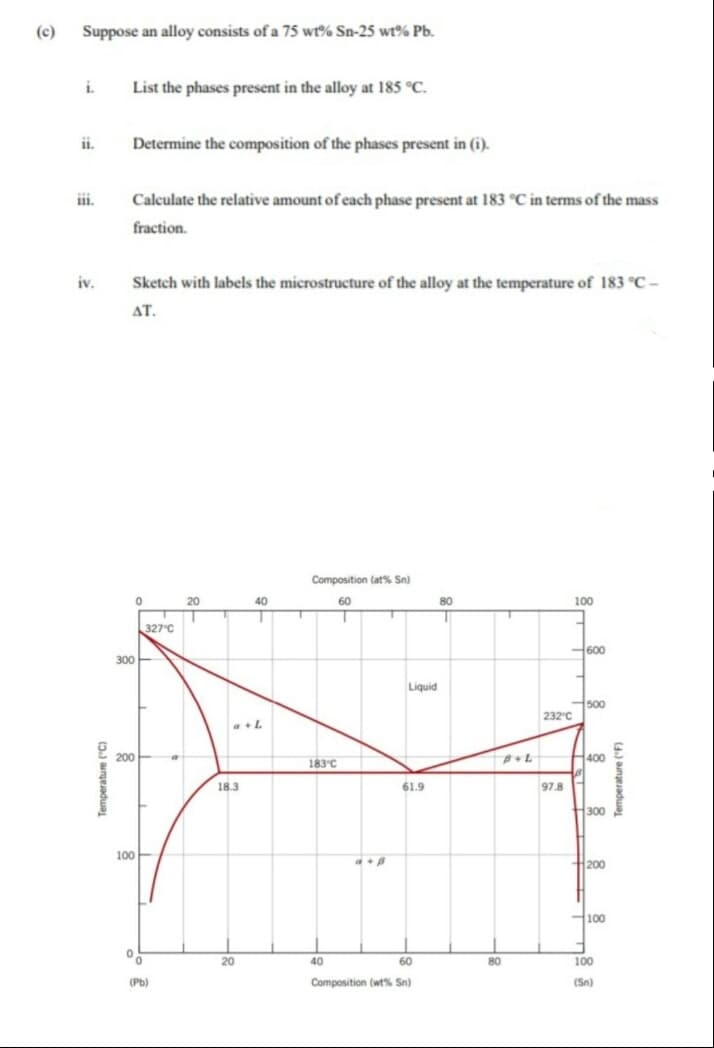
Transcribed Image Text:(c)
Suppose an alloy consists of a 75 wr% Sn-25 wt% Pb.
i.
List the phases present in the alloy at 185 °C.
i.
Determine the composition of the phases present in (i).
iii.
Calculate the relative amount of each phase present at 183 °C in terms of the mass
fraction.
iv.
Sketch with labels the microstructure of the alloy at the temperature of 183 °C -
AT.
Composition (at% Sn)
20
40
60
80
100
327°C
600
300E
Liquid
500
232 C
a+L
200
183°C
400
18.3
61.9
97.8
300
100
200
H100
20
40
60
80
100
(Pb)
Composition (wt% Sn)
(Sn)
Temperature ("C)
4.) aungesadua
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning