Calculate the binding energy of Al-28, and the transition energy of the Al-28/Si-28 beta-minus decay. Al-28 protons neutrons electrons component mass number amu 1.007277 1.008665 0.000549 total
Calculate the binding energy of Al-28, and the transition energy of the Al-28/Si-28 beta-minus decay. Al-28 protons neutrons electrons component mass number amu 1.007277 1.008665 0.000549 total
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter18: Nuclear Reactions
Section: Chapter Questions
Problem 82QAP
Related questions
Question
Please do it per the outline on the paper.
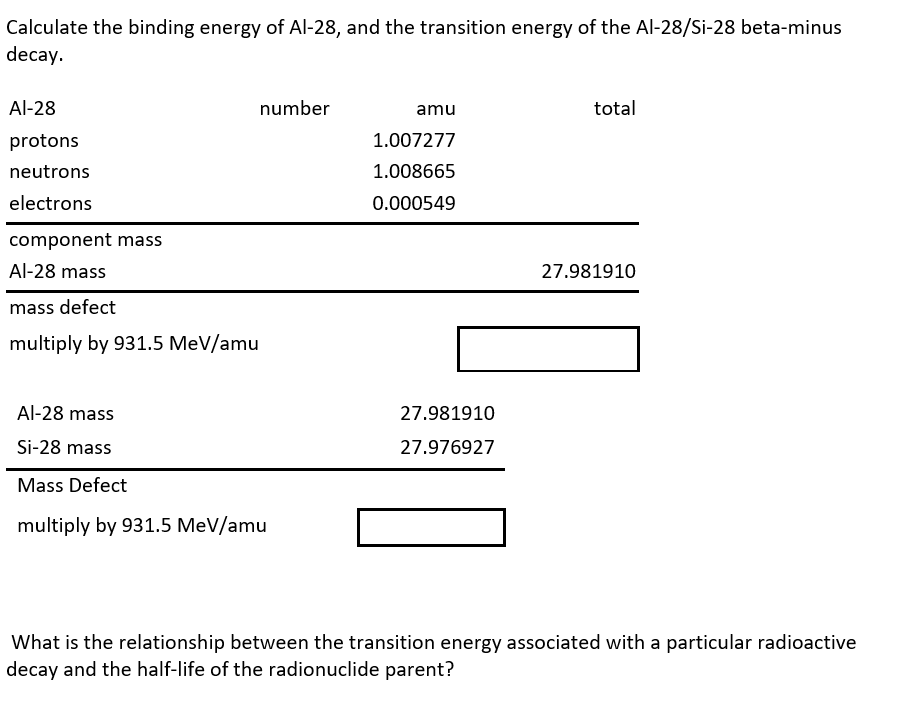
Transcribed Image Text:Calculate the binding energy of Al-28, and the transition energy of the Al-28/Si-28 beta-minus
decay.
Al-28
protons
neutrons
electrons
component mass
Al-28 mass
mass defect
multiply by 931.5 MeV/amu
number
Al-28 mass
Si-28 mass
Mass Defect
multiply by 931.5 MeV/amu
amu
1.007277
1.008665
0.000549
27.981910
27.976927
total
27.981910
What is the relationship between the transition energy associated with a particular radioactive
decay and the half-life of the radionuclide parent?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning