Q: In terms of mass, calculate the percentage of the active ingredient for each of the following…
A: Mass percentage is defined as the total mass of the substance( here active ingredients) divided by…
Q: What mass of salt (NaCl) in grams (g) is required to prepare 2.013 L of D51/4NS solution? Give your…
A: Given the volume of D51/4NS solution = 2.013 L. D51/4NS solution has 0.225% saline (NaCl) and 5.0%…
Q: How many grams of sucrose are needed to make 915 mL of a 32.0% (w/v) sucrose solution? mass: g…
A:
Q: How many moles of NaOCI would be present in 1 gallon of commercial bleach (density = 1.05 g/ml),…
A:
Q: bnuot nasd OL por 2. Seven hundred fifty milliliters (750 mL) of a well-known alcoholic beverage is…
A: Given: Volume of solution i.e. beverage = 750 mL. And the concentration of ethanol = 55.0 proof.
Q: Calculate the percent composition of sand in the mixture using the mass of the recovered…
A: Hello. Since you have posted multiple questions and not specified which question needs to be solved,…
Q: The sulfur in an 8-tablet sample of the hypnotic drug captodiamine, C21H29NS2 (359.6 g/mol) was…
A: Given : Molar mass of captodiamine = 359.6 gm/mol Mass of BaSO4 recovered = 0.3343 g molar mass…
Q: (a) Palladium (Pd) is an element with properties similar to those of platinum. It is useful in…
A: Given : Mass of bar = 96.03 g Length of bar = 10.7 cm Diameter of bar = 9.82 mm = 0.982 cm…
Q: In the Dumas Method for the determination of the molar mass of an unknown vapor, the weight of the…
A: Given, Weight of the flask filled with water = 378.56 g Weight of the water = 252.68 g Density of…
Q: Using the available data in the graph (below), calculate the percent by mass of Potassium Bromide in…
A: Percentage by mass is a method to represent the concentration of any solution, and it s defined as…
Q: A student needs exactly 25.0 mL of a 1.5M solution of NaOH (molar mass: 40.00 g/mol). They have…
A: Given: Concentration of NaOH = 1.5 M Volume of solution required = 25.0 mL = 0.025 L…
Q: 1.A student performed an experiment to find the number of water molecules associated with the CuSO,…
A: Since you have posted questions with multiple subparts, we will only first three subparts for you.…
Q: calculate volume H2=Volume displaced water
A: Given Mass of Empty Beaker is 141.2g, Beaker + Water is 388.3g, mass of water is 247.1g, volume of…
Q: Vinegar is a solution of acetic acid (the solute) in water (the solvent) with a solution density of…
A: Given : Concentration of vinegar = 0.80 M And density of vinegar solution = 1010 g/L Assuming 1 L of…
Q: A solution of 20.91 % by weight H2SO4 has a specific gravity of 1.150. What is the normality of this…
A: Answer-1 Specific gravity is the ratio of density of a substance with the density of water. Since,…
Q: A student found that her mixture was 13% NH4Cl, 18% NaCl and 75% SiO2. Assuming that her…
A: Given In mixture % of NH4Cl = 13% % of…
Q: A chemist adds 25.0 mL of a 0.0011 mol/L copper(II) fluoride (CuF,) solution to a reaction flask.…
A:
Q: On 12 and 13 I got a negative scientific on one and a positive on the other. Did I make a mistake…
A: General chemistry12. The number of atoms or molecules in a mole of any substance is equal to…
Q: Whilst working in the pharmacy aseptic unit you are asked to prepare 5 litres of a solution…
A: 1 mmol/mL = 1 mol/L = 1 M This means 1 L of this solution contains 1 mole KCl So, to prepare 5 L…
Q: A pycnometer is a glass flask with a tight fitting glass stopper that can be used to determine the…
A: A pycnometer is an apparatus that can be used to determine the density of an insoluble solid by…
Q: In an experiment involving the extraction of essential oils from a plant, 7.5 mL of benzene (C6H6,…
A: Volume of benzene = 7.5 mL Density of Benzene = 0.8765 g/mL Mass of Benzene = Volume * Density…
Q: 3. A student forgot to pre-measure the mass of the second evaporating dish prior to decanting the…
A: Since you have asked multiple question, we will solve the first question for you. If you want any…
Q: Question attached
A: The given data contains, Density of water =1.000g/cm3 Mass of clean pycnometer =25.151 g Mass of…
Q: A mixture is made by combining 1.02 lb of salt and 3.76 lb of water. What is the percentage of salt…
A: Percentage of salt (%) by mass is given by percentage by mass = mass of salt x 100/mass of water…
Q: Vinegar is a solution of acetic acid (the solute) in water (the solvent) with a solution density of…
A: Given : Concentration of vinegar = 0.80 M And density of vinegar solution = 1010 g/L Assuming 1 L of…
Q: What mass of salt (NaCl) in grams (g) is required to prepare 4.647 L of D51/4NS solution? Give your…
A: D5 1/4 NS solution means D5 0.45 NS => 5% Dextrose in 0.225% Saline (D5 1/4NS)
Q: A pycnometer is a glass flask with a tight fitting glass stopper that can be used to determine the…
A: The ratio of mass to the volume is known as density.
Q: How many grams of water must be used to prepare a 2.50 percent by mass (%m/m) sodium hydroxide…
A:
Q: When answering this problem, report the answer with the appropriate number of significant figures.…
A: Solution - According to this question - Given - Formula - no of mole = molarity x volume thus, no of…
Q: You record the following data during the Qualitative and Quantitative Characterization of Aspirin…
A: A numerical problem based on concentration terms, which is to be accomplished.
Q: Commercial solvents are commonly sold in 55-gallon drums. What is the weight in pound of the carbon…
A: Volume of the solvent = 55 gallon Volume of solvent in cc = 208198 cm3 (since 1 gallon = 3785.41…
Q: calculate the mass percent of your solution. show your calculations
A: Mass percent is the physical quantity used to define the concentration of the species Mass percent…
Q: Instructions: solve as neatly as possible and show complete solution. all values must include…
A:
Q: Equivalence can be calculated by: (tick all that apply) L multiplying normality with volume in…
A: Equivalents are defined in such a way so that in any chemical reaction equivalents of reactant and…
Q: An alcoholic fermentation was conducted in the laboratory using 280 g glucose as substrate with a…
A: Formula used are given below: Density = Mass of substance / Volume of substance and Percent Volume =…
Q: Density = (0.9M x 36.46g/mol)/36 = 32.814/36 = 0.9115g/mL My problem is…
A:
Q: During an experiment you extract 112 mg of caffeine from a 16g sample of coffee. What is the mass…
A: A) Given: The mass of caffeine in coffee = 112 mg = 0.112 g ( Since 1 mg = 0.001 g) The mass of…
Q: As part of the aspirin synthesis lab the orgo students also had to perform the following calculation…
A: For determining the amount of product, the mole ratio of reactants in a balanced reaction must be…
Q: Carbon dioxide (CO2) is the gas that is mainly responsible for global warming (the greenhouse…
A:
Q: 750 g Oral 237 mL per bottle Potassium 950 g elemental iodine + 1.5 kgs potassium hydroxide…
A: Given that - Potassium iodide ,KI Here only one Compound is given , which is potassium iodide, KI…
Q: essential oils from a plant, 7.5 mL of benzene (C6H6, 78.11 g/mol) was mixed with 25.00 mL of hexane…
A:
Q: The three sets of units for expressing density (or concentration, as in “mass of solute per unit…
A: The density is the mass mass of solute per unit volume of solvent. Therefore, it can be expressed in…
Q: Borax (boron), Na2B4O7.10H2O (Sodium tetraborate decahydrate, the 10 in front of the water means…
A: Given, Borax (boron), Na2B4O7.10H2O
Q: Use the References to access important values if needed for this question. Calculate the number of…
A:
Q: A fictional cubed-shaped bacterium, Bacterius cubis, occupies a volume of 2.8 femtoliters. This…
A:
Q: A solution of 20.91 % by weight H2SO4 has a specific gravity of 1.150. What is the normality of this…
A: We have to find normality of solution
Q: essential oils from a plant, 7.5 mL of benzene (C6H6, 78.11 g/mol) was mixed with 25.00 mL of hexane…
A:
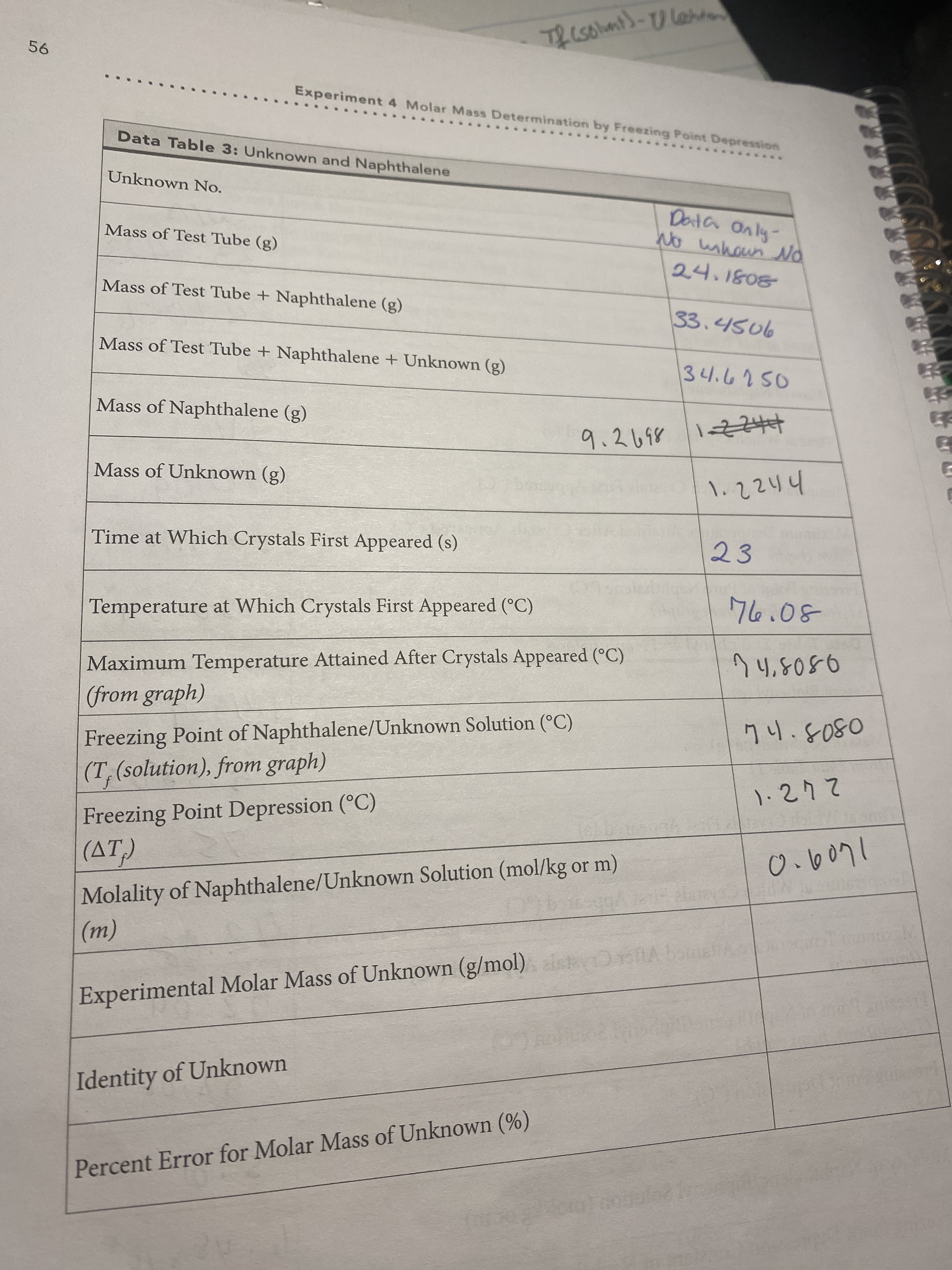
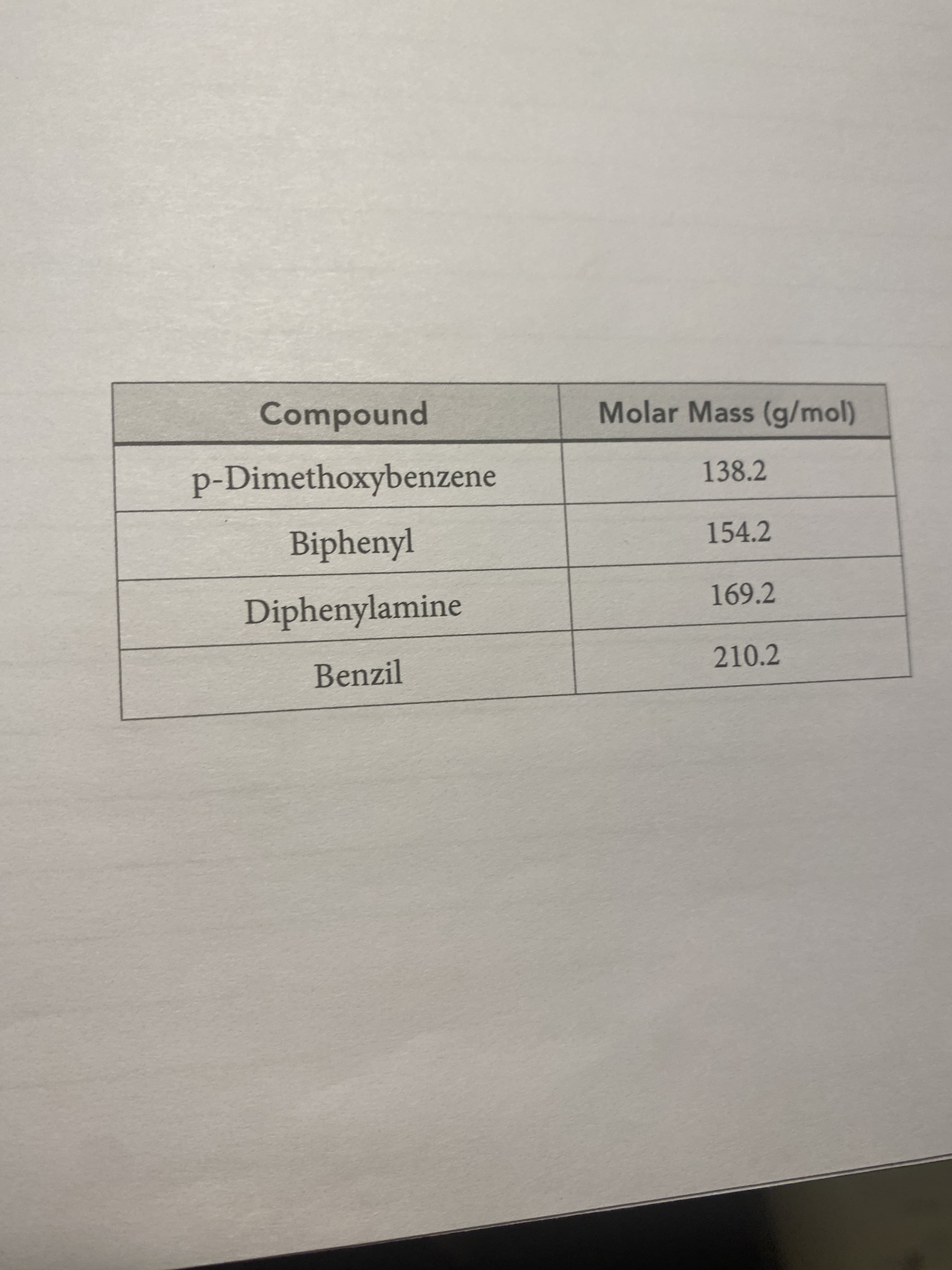
Calculate the molar mass of the unknown. Use the chart to determine the identity of the unknown based on the calculated experimental molar mass of compound
Mass of tube 24.1808(g)
Mass of test tube +Naphthalene (g)=33.4506
Mass of test tube+ naphthalene +unknown (g) =34.6750
Time when crystals first appeared seconds = 23 C
Temperature whiv crystals first appeared 76.08 C
Molarity of naphthalene/ unknown solution = .6071
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- density of irregular solid mass of irregular solid 9.039 g initial volume 68 mL final volume 74 mL total volume displaced 6 mL what is the density of irregulatr solid?An experiment was performed to look at how density changes when t he ratio of water and ethanol was changed. The following data was obtained: % Mass Ethano1.00 2.50 3.50 4.00 6.00 7.50 9.50 Density, g/mL0.9963 0.9936 0.9918 0.9910 0.9878 0.9855 0.9826 Use Excel or a similar spreadsheet program to plot these six data points and answer the following questions . a.What is the independent variable? b.What is the dependent variable? c.What is the equation of the best-‐fit line? d.What is the value of the coefficient of determination? e. According to the best-‐fit line, what should the density of pure water be (i.e. what is the density when % mass ethanol = 0.00)?An experiment with a pycnometer resulted in the following data: Mass of Pycnomter = 25.428 g Mass of Pycnometer filled with water = 44.021 grams Mass of Pycnometer filled with liquid X = 41.421 grams a) What is the specific gravity of liquid X? b) If methanol (Specific Gravity = 0.790) was used rather than water in measuring the density of irregular objects, how would that affect the result? Why?
- In order to find the density of a solution of unknown density, 10 mL of solution was drawn into a clean and dry beaker three times with a pipette and weighed and the weighing results were found to be 65.1452 g, 64.9982 g, 65.1027 g. Calculate the density of the solution and the standard deviation of the measurements, since the empty weight of the beaker is 52.2461 g.Please answer this question as fast as you can please and tahnk you. I will afterwards write an wonderful review on solving the question. Thank you. For each piece of glassware measure the density of water in triplicate (three times). Why repeat the procedure multiple times for each piece of glassware? Running the measurement in triplicate increases the precision of our results and reduces the impact of a single outlier result. The experiment would be too short if you only ran each trial once The first two trials act as practice, and the final trial will give you the true result. Running the measurement in triplicate increases the accuracy of our results and reduces the impact of a single outlier result.You are assigned the task of separating a desired granular materialwith a density of 3.62 g/cm3 from an undesired granularmaterial that has a density of 2.04 g/cm3. You want to dothis by shaking the mixture in a liquid in which the heaviermaterial will fall to the bottom and the lighter material willfloat. A solid will float on any liquid that is more dense. Usingan Internet-based source or a handbook of chemistry, findthe densities of the following substances: carbon tetrachloride,hexane, benzene, and diiodomethane. Which of theseliquids will serve your purpose, assuming no chemical interactiontakes place between the liquid and the solids?
- A pycnometer is a glass flask with a tight fitting glass stopper that can be used to determine the density of an insoluble solid by measuring the volume of water ejected out of the pycnometer when sample of the solid is placed in it. Determine the density in g/cm³ of an unknown metal alloy sample from the following measurements. Assume the density of water is 1.000 g/cm³.Mass of clean pycnometer = 25.151 gMass of clean pycnometer plus water = 42.481 gMass of clean pycnometer plus metal sample = 39.305 gMass of clean pycnometer plus metal plus water = 54.265 g (after ejected water is dried from the outside surface)How many milliosmoles (mOsm) are there in 0.710 Osm? How many square centimeters (cm2) are there in a square kilometer (km2)? A plane travels at Mach 2.5, how long will it take to travel from California to Germany (9112 km)?At 20oC 25.0 ml of concentrated nitric acid solution was found to have a mass of 32.503 g. What is the calculated density of this solution at 20oC? Show your calculation set-up and result using correct significant figures and proper units. Volume of Nitric acid = 25.0 ml Mass of nitric acid = 32.503 g Density = mass of substance Density= Mass of nitric acid volume of substance volume of nitric acid = 32.503g 25 .0mL = 1.30g/mL (3 sig figures) Using the calculated density of the solution from above, what is the mass of 150 ml. of the solution? Again show your solution set-up with correct significant figures and units.
- A group of students is doing an analysis of copper in a 1-peso coin. They were tasked to prepare standard solutions and weigh the analyte as the end-product of their experiment. What type of error is it and explain how this type of error incurring in their experiment. a. Makisig forgot to calibrate the analytical balance before weighing. b. Only one trial was performed in the experiment. c. One member inadvertently recorded 29.90 mL instead of 19.90 mL volume. d. Marikit had very good precision but poor accuracy in her data.e. Volumetric flasks were placed inside the oven for drying before it was used.f. Trial 1 and 2 have values close to 30.0 mL while trial 3 gave 55.0 mL.g. The reagent used in determining copper was added incorrectly and gave a low yield.16 oz = 1lb 200lb=1T 1oz =28 g 1lb= 0.45 kg 1T=0.9t 71lb to g 71lb =___ g using the given, equivalence, along with dimensional analysis, to convert the given unit to the unit indicated.Given the following data for Mass of test tube and stearic acid = 14.17 gMass of test tube = 11.40 gFreezing point of strearic acid = 69.59o CMass of weighing paper + naphthalene =1.230 gMass of weighing paper = 0.920 gFreezing point solution = 64.00o CKf = 4.5o C/m Determine the following1. mass of stearic acid in g (2 decimal places); 2.77g2. mass of naphthalene in g (2 decimal places); 0.31g3. freezing point depression (2 decimal places); 3.93oC4. molality of solution (3 significant figures); _____5. moles of naphthalene (3 significant figures); _____6. molar mass of naphthalene, experimentally (3 significant figures); _____7. % error if theoretical molar mass of naphthalene is 128.17 g/ mole, USE ABSOLUTE VALUE (3 significant figure); _____

