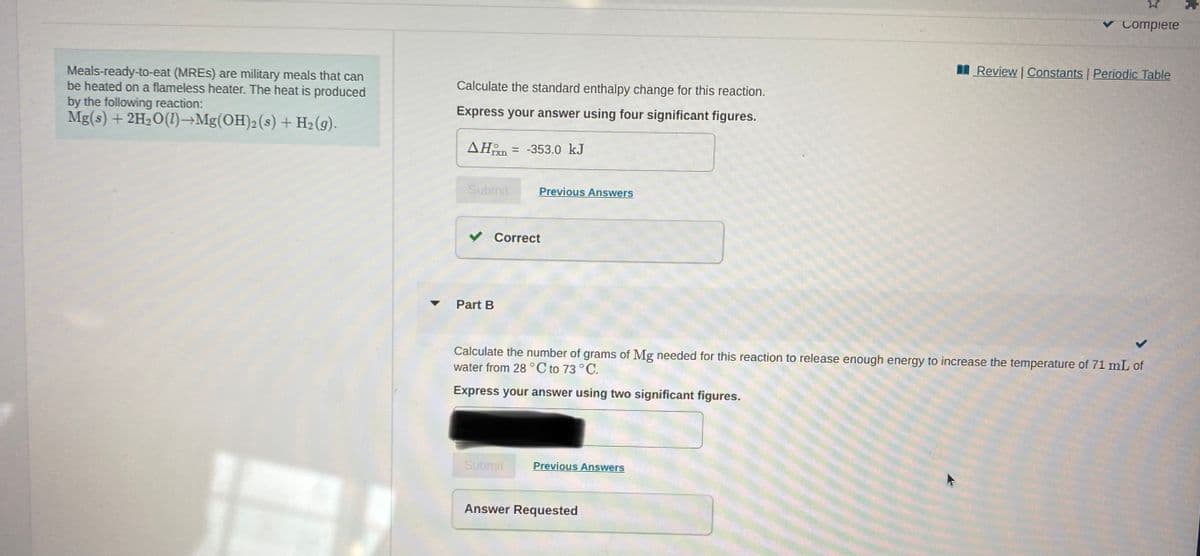
Calculate the number of grams of Mg needed for this reaction to release enough energy to increase the temperature of 71 mL of water from 28°C to 73°C. Express your answer using two significant figures.
Calculate the number of grams of Mg needed for this reaction to release enough energy to increase the temperature of 71 mL of water from 28°C to 73°C. Express your answer using two significant figures.
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 94E: The complete combustion of acetylene, C2H2(g), produces 1300. kJ of energy per mole of acetylene...
Related questions
Question
Can you explain Part B?
Transcribed Image Text:v Compiete
I Review | Constants Periodic Table
Meals-ready-to-eat (MRES) are military meals that can
be heated on a flameless heater. The heat is produced
by the following reaction:
Mg(s) + 2H2O(1)→Mg(OH)2(s) + H2(g).
Calculate the standard enthalpy change for this reaction.
Express your answer using four significant figures.
AHn
= -353.0 kJ
Submit
Previous Answers
Correct
Part B
Calculate the number of grams of Mg needed for this reaction to release enough energy to increase the temperature of 71 mL of
water from 28 °C to 73 °C.
Express your answer using two significant figures.
Submit
Previous Answers
Answer Requested
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning