Calculate the pH of a solution that is 0.0510 M in а. Н3РОД (Ка1 — 7.1 х 10 3). pH = b. НзРОз (Кal = 3.0 x 10-2). pH = с. Н2S (Ka1 — 9.6 х 10 $). pH = %3D
Calculate the pH of a solution that is 0.0510 M in а. Н3РОД (Ка1 — 7.1 х 10 3). pH = b. НзРОз (Кal = 3.0 x 10-2). pH = с. Н2S (Ka1 — 9.6 х 10 $). pH = %3D
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter15: Complex Ion And Precipitation Equilibria
Section: Chapter Questions
Problem 10QAP: Calculate [OH-] and pH in a solution in which the hydrogen sulfite ion, HSO3-, is 0.429 M and the...
Related questions
Question
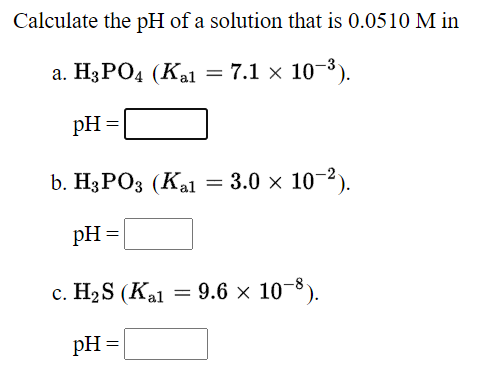
Transcribed Image Text:Calculate the pH of a solution that is 0.0510 M in
а. H3РОД (Кa — 7.1 x 10 -3).
pH =
b. Н3РОЗ (Каl
= 3.0 x 10-2).
pH =
с. НаS (Kal
= 9.6 x 10-8).
pH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning