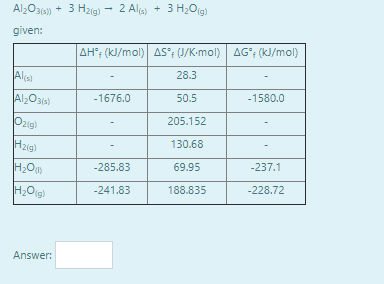
Calculate the standard free energy for the reaction (you may use any valid technique - there may be more than one way to do a question like this):
Q: Try Again Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction…
A: Standard free energy of reaction can be calculated using standard free energy formationof reactants…
Q: Consider the reaction: 2H,0,(1)2H,0M + O,(g) Using standard thermodynamic data at 298K, calculate…
A: E0 (H2O2/H2O) =1.776 V E0 (H2O2/O2)=-0.68V E0 cell = 1.776 +(-0.68) =1.096V ∆G rxn=-nFE0 cell…
Q: sider the reaction: 2NH3(g) + 2O2(g)N2O(g) + 3H2O(l) Using standard thermodynamic data at 298K,…
A: The question is based on the concept chemical thermodynamics. We have been given an equation. We…
Q: Calculate the free-energy change of the following reaction at 410°C and standard pressure. Values in…
A: In thermodynamics, Gibbs free energy is a thermodynamic potential that can be used to calculate…
Q: What is the main reason why ΔG or change in free energy is important for determining the spontaneity…
A: ∆G° is the Gibbs free energy which tells about the spontaneity of the reaction. If ∆G° = +ve…
Q: Consider the reaction: C2H4(g) + H2O(g)→CH3CH2OH(g) Using standard thermodynamic data at 298K,…
A: The expression for change in energy is shown below: where; ∆Gproducts = change in Gibb's free…
Q: Consider the reaction: 2Na(S) + 2H-0()—2NaОН(аq) + H2(9) Using standard thermodynamic data at 298K,…
A: Given data,∆GofH2O(l) = -237.1 kJ/mol ∆GofNa(s) = 0 kJ/mol ∆GofNaOH(aq) = -419.2 kJ/mol ∆GofH2…
Q: Write the equation that relates the change in free energy, ?G, to ?H and ?S. What does it mean if a…
A: Equation relates the change in free energy = To be determined Spontanety of reaction = to be…
Q: Answer the following questions to the best of your knowledge. a. Why the Gibb’s free energy is such…
A: A state function is the property of the system that depends only on the state of the system. It…
Q: 7a. Calculate the value of AG° for the reaction below, given the values of AH° and AS°. Will the…
A: Given a chemical reaction Cu2S + S -> 2cus Given of data a ΔH° = -26.7kj/mole ΔS° =…
Q: The standard free energy of formation of solid sodium bromide is -347 KJ/mole. The standard Gibb’s…
A:
Q: Why does the Gibb’s free energy is such a useful state function?
A: To find: Gibbs free energy is such a useful state function.
Q: Consider the reaction 2H,O1) 2H,(g) + 0,(g) The standard free energy change for this reaction is…
A: The amount of energy released during the conversion of reactant into the product under the standard…
Q: Consider the reaction: 4HCI(g) + O2(g)–2H,0(g) + 2Cl,(g) Using standard thermodynamic data at 298K,…
A: Moles of HCl = 1.84 mole 4HCl + O2 -> 2H2O + 2Cl2
Q: AS=- 2550 J/mole.K, at what erature range would this on be spontaneous?
A: Given , □H = 150 kJ/mol □S = -2550 J/K For Spontaneousreaction, □G <0 □G = □H - T □S (□H - T…
Q: Using the values for standard Gibb's Free Energy in the table, calculate the Gibb's Free Energy for…
A: Given reaction: A + 3B = 2C + D Free energy (Gibbs free energy) is the term that is used to explain…
Q: A reaction has values of AH and AS which are both positive. The reaction * Is spontaneous O…
A: When ∆H and ∆S both are positive then reaction is ---
Q: Consider the reaction: 3Fe,0,(s) + H₂(g)-2Fe₂O₂(s) + H₂O(g) Using standard thermodynamic data at…
A:
Q: show by calculation which direction the reaction would be energetically spontaneous, use…
A: The given reaction is as, Silicon Atomic number of Si = 14 Number of protons = 14 Number of…
Q: Consider the reaction: 2NH3(g) – 20,(g)–→N;O(g) + 3H,O() Using standard thermodynamic data at 298K,…
A: Given information: Moles of ammonia = 1.82 mol
Q: Consider the following reaction at 25 °C: 3 Ni(s) + N₂(g) + 3 H₂O(g) → 3 NiO(s) + 2 NH₃(g) If ∆H° =…
A: We are having the value of ∆H° = -85.7 kJ/mol and ∆S° = -348.3 J/mol.K We have to calculate the…
Q: onsider the reaction: 2HBr(g)H2(g) + Br2(l) Using standard thermodynamic data at 298K, calculate…
A: Given : Moles given = 1.58 moles
Q: The standard free energy (DGo) for a reaction at 298 K is -104 J / mol. What is Keq for this…
A: Given, The standard free energy (DGo) for a reaction at 298 K is -104 J / mol.
Q: For the reaction 2Na(s) + 2H2O(l)2NaOH(aq) + H2(g) H° = -368.6 kJ and S° = -15.3 J/K The standard…
A: Given reaction: 2Na(s) + 2H2O(l)-------> 2NaOH(aq) + H2(g) H° = -368.6 kJ = -368600 J S° = -15.3…
Q: Which of the following does the change in the free energy of a reaction predict?
A: Relation between free energy change, enthalpy change and entropy change is shown below: ∆G=∆H-T∆S
Q: The value of AG° for the reaction 2C4H10 (9) + 1302 (9) → 8CO2(9) + 10H,0(1) is -5490. kJ. Use this…
A: The balanced reaction taking place is given as, => 2 C4H10 (g) + 13 O2 (g) -------> 8 CO2 (g)…
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A: Given reaction P4(s) + 20HF(g) → 4 PF5 (g) + 10 H2(g)Standard reaction free energy = To be…
Q: spontaneous or non-spontaneous.
A:
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A:
Q: Consider the reaction:C2H4(g) + H2O(g)CH3CH2OH(g)Using standard thermodynamic data at 298K,…
A: The standard thermodynamic data for the given reactants and products at given temperature is,
Q: d) At what temperatures would this reaction be spontaneous? [Select]
A: The reaction given shows heat is emitted during the reaction. Answer to part d (as asked) is being…
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A: Free energy is that portion of any first-law energy that is available to perform thermodynamic work…
Q: Consider the reaction: 2H,S(g) + 302(g)→2H,O(g) + 2SO,(g) Using standard thermodynamic data at 298K,…
A: The standard free energy for the chemical species that are involved in the reaction is shown below.
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A: The given balanced chemical reaction is, 2Als+Fe2O3s→Al2O3s+2Fes The oxidation half reaction is…
Q: Using the thermodynamic information, calculate the standard reaction free energy for the reaction…
A:
Q: A spontaneous reaction has _________ deltaG and the potential for such reaction is _______________
A: For any reaction to be spontaneous, it has to have -ve delta G value. Hence A spontaneous reaction…
Q: of 2 mM. Under these conditions calculate the actual free energy (AG) of the reaction of
A: the free enrgy is calculated by the formula,ΔG=ΔG0 + RT ln Keqwhere,ΔG0 =standard free energyR=gas…
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A: From ALEKS data tab: ΔG0 for H2 = 0 KJ.mol-1 ΔG0 for O2 = 0 KJ.mol-1 ΔG0 for H2O = 228.57 KJ.mol-1
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A:
Q: Consider the reaction: 2C,H6(g) + 702(g)4CO2(g) + 6H,0(g) Using standard thermodynamic data at 298K,…
A:
Q: Consider the reaction: 2C,H6(g) - 702(3)4CO2(g) + 6H,0(g) Using standard thermodynamic data at 298K,…
A: Given information: Moles of C2H6 = 1.77 mol
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A: Given C(s) + 2Cl2 (g) →CCl4 (g)
Q: Which of the following does the change in the free energy of a reaction predict? A) the work done B)…
A: Free energy is a deciding factor for the spontaneity of any chemical reaction. Lower free energy of…
Q: Which of the following statements determine a reaction to be spontaneous? O The ASpxn is positive. O…
A: The relation between change in entropy, enthalpy, and Gibbs free energy can be given as: ∆G=∆H-T∆S…
Q: Assuming the change in free energy is zero at the boiling point of water, calculate the ΔS of the…
A: Given : Free energy change i.e ΔG is 0 at boiling point of water i.e 100 oC or 373 K.
Calculate the standard free energy for the reaction (you may use any valid technique - there may be more than one way to do a question like this):
Step by step
Solved in 3 steps with 2 images

- 15 Calculate ΔGrxn in (kJ/mol) for the reaction for which ΔHrxn=-26.1kJ/mol ; ΔS=3.89J/molK at 25oC Group of answer choices A, -27.3 B, -24.9 C, 24.9 D, -26.2 E, 28.35 16.Using the values forΔH° and ΔS°provided in Appendix G calculate ΔG°rxn in (kJ/mole) for the following reaction. 2 C6H6(l) + 15 O2(g) →12 CO2(g) + 6 H2O(l) Group of answer choices A, -6290 B, -6404 C, -6390 D, -6505Calculate ΔG. For the following process at 25°C: BaF2 (s) = Ba2 + (aq) + 2F - (aq) The Ksp of BaF2 is 1.7 x 10^-6 ΔG° = _____ kJ/molDifferentiate between circumstances for the use of nRln(V2/V1) ??? Cvln(T2/T1) in calculating change in S
- The density of ethane is 545 kg/m3. If its specific energy is found out to be -86.8 MJ/kg, what is the energy density of ethane? Use approximation.G.192. With explanation..can someone find the [OH-] at T hot, the Ksp at T hot, the delta G for T hot, the delta S and delta H and % error given the following values Temp Room: 20.1 deg Cel Temp Hot: 30.7 deg Cel pH Room: 12.78 pH Hot: 12.44 calculate the value given the following standard state entropies S f Ca(OH)2 (aq) = -74.6 J/mol K S f Ca(OH)2 (s)= 83.39 J /mol K
- Using ∆Hof from the table, calculate ∆Hrxn for: Pb (s) + PbO2 (s) + 2 SO3 (g) → 2 PbSO4 (s) substance Pb (s) PbO2 (s) SO3 (g) PbSO4 (s) ∆Hof 0 kJ/mol -277 kJ/mol -395 kJ/mol -920. kJ/mol1. Use the following reactions to determine the Delta Hrxn for P4O10(s) + 6H2O(l) 4H3PO4(l) 4P(s) + 5O2(g)---> P4O10(s). Delta H = -2984kJ 2H2(g) + O2(g)---> 2H2O(l) Delta H = -570kJ 2P(s) + 4O2(g) + 3H2(g)---> 2H3PO4(l). Delta H = -2534kJ B. Is the reaction exothermic or endothermic? C. Given Sof P4O10(s) = 228.9 J mol-1 K-1; Sof H2O(l) = 69.9 J mol-1 K-1; Sof H3PO4(l) = 241.98 J mol-1 K-1 calculate the DGrxn at 28.0oC. D. Is this reaction spontaneous or nonspontaneous?Calculate Ksp for the salt NaCl at 25°C. Substance ΔGf°(in kJ/mol) Na+(aq) –262.0 Cl–(aq) –131.0 NaCl(s) -383.6
- Thermodynamics Quantities for Selected Substances at 298.15 K (25⁰C) Substance ∆H⁰f (kJ/mol) ∆G⁰f (kJ/mol) S (J/K-mol) Hydrogen H2(g) 0 0 130.58 Oxygen O2(g) 0 0 205.0 H2O(l) -285.83 -237.13 69.91 10. What is the ∆S⁰ in the combustion of hydrogen in the presence of excess oxygen yields water: 2H2(g) + O2(g) → 2H2O(l) in J/K?Given the following processes and their respective enthalpies in kJ/mol: Li(s) --> Li(g); DHs = +161 Li(g) --> Li+(g) + e–; IE1 = +520 F2(g) --> 2F(g); BE = +160 F(g) + e– --> F-(g); EA = -328 Li+(g) + F-(g) --> LiF(s); UL = x If the reaction: Li(s) + ½F2(g) --> LiF(s) has DHf= -616.0 kJ/mol, deduce the value of x for UL (lattice energy in kJ/mol) in LiF. (A) -1129 (B) -1049 (C) -183 (D) -103Calculate ΔG0 (in kJ/mol) given ΔG= -423.1 kJ/mol and R= 0.008314 kJ/mol K and T= 611.3 K and Q=0.601ΔG=ΔG0+RTlnQ

