Q: Calculate the pressure to which this volume of air must be compressed in order to fit into the air…
A: Calculate the pressure.
Q: I don't know where to start.
A: Using the given data, graph can be drawn by plotting pressure against volume and is given below:
Q: Torricelli, who invented the barometer, used mercury in its construction because mercury has a very…
A:
Q: Describe in your own words, the contributions of each of the following scientists with respect to…
A: Boyle's law: At constant temperature and for a fixed amount of an ideal gas, pressure of the gas is…
Q: How will the unknown’s reported molar mass be affected in each of the following cases The…
A: Since you have asked a question with multiple sub-parts, we will solve first three sub-parts for…
Q: To calculate universal gas constant (R) experimentally, the thermal decomposition reaction of KClO3…
A: The thermal decomposition reaction of KClO3 gives KCl and oxygen. MnO2 is used as catalyst for the…
Q: Calculate the hydrostatic pressure in units of atmospheres for a closed end manometer with a mercury…
A: Fluid statics or hydrostatics is the branch of fluid mechanics that studies "fluids at rest and the…
Q: What additional information must be considered to determine the number of moles of gas produced? The…
A: The numbers of moles calculation we require VOLUME , PRESSURE and TEMPERATURE. Volume of gas can be…
Q: using GRESA format What would happen to the pressure of a 2.00 L oxygen gas (at standard temperature…
A: Given Volume at STP = 2 L Volume change = 473 L At STP pressure will be 1 atm or 760 mmHg
Q: Assuming standard condition, what is the altitude in km with the given pressure of 10.51334141 cm…
A: Concept - altitude means the height of something above sea level. so here the pressure is due to…
Q: *** 21 - Calculate R from student collected data and percent error A student weighs out 0.0422 g of…
A:
Q: Pressure conversion: Convert 30.03 inches Hg to atmospheres. (Hint: First convert to cm Hg, then to…
A: Given : 30.03 inches Hg To find : Convert it to atmospheres Solution: Pressure can have many units…
Q: Part A: Calculate the molar mass of a gas that has a density of 1.98 g/L at STP. Part B:…
A: To Solve this problem we use the relation that d = PM/RT Where , d = density P = pressure M =…
Q: A hot-air balloon will rise when the density of its air is 10.1% lower than that of the atmospheric…
A: Given: Temperature = 319.7 K Pressure = 0.9 atm Composition of nitrogen = 78% composition of oxygen…
Q: When a certain free element (which is solid at room temperature) is heated, it forms vapor molecules…
A:
Q: column of mercury exerts a pressure of 151, 988 Pa. Calculate the height of this column of mercury.
A: According to the question, We have pressure exerted by the mercury column = 151988 Pa We need to…
Q: A student reads a barometer in the laboratory and finds the atmospheric pressure to be 793.6 mmHg.…
A: Given : Pressure= 793.6 mmHg To find: The conversion of pressure into atmospheres.
Q: How are the properties of real gasses taken into account? Group of answer choices a.By applying an…
A: Since the ideal gas assumes that there is no force of attraction between the molecules and the…
Q: A student weighs out 0.0422 g of magnesium metal. The magnesium metal is reacted with excess…
A: The balanced reaction of Mg with HCl is as follows: Mg(s)+2HCl(aq)→MgCl2(aq)+H2(g) Calculation of…
Q: A sample of gas is collected in a balloon that can easily expand and contract. It should be noted…
A: An ideal gas is one that obeys ideal gas equation at all temperatures and pressures. A perfect ideal…
Q: 21. The temperature of Oxygen is changed from 110°F to 270°F while pressure remains constant.…
A: K = (F-32)/1.8 +273.15 (110-32)/1.8+273.15 = 316.48K (270-32)/1.8+273.15=405.37K PV= nRT ideal gas…
Q: Determine the amount of oxygen gas in a room whose dimensions are 4m × 5m × 6m at 100kPa and 25°C.…
A: The molar mass (MMO2) of the oxygen gas is 32.0 g/mol. The volume of the room is; 1 atm=101.3 kPa100…
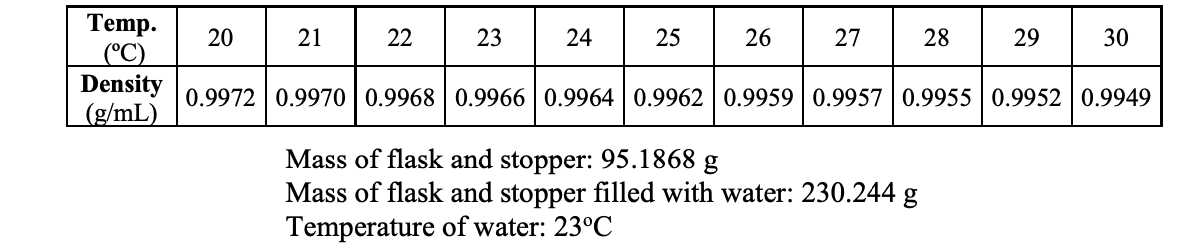
Q: Calculate the volume of the flask using the mass of water which filled the flask and the density of…
A: Answer in table Temperaure (°C) 20 21 22 23 24 25 26 27 28 29 30 Density (g/mL) 0.9972 0.9970…
Q: Distinguish between a "wet" gas and a "dry" gas. Drag the appropriate features of a "wet" gas and a…
A: The given points are: (1) The pressure exerted bt it is equal to the pressure of its components plus…
Q: What is the temperature in °C, of 5.50 mol of a gas that occupies a volume of 100.5L at a pressure…
A:
Q: Calculate the pressure of air at 81.1 C (354.3K) using the value 10.78 moles. Assume a corrected…
A: In order to find the pressure we first write down the given data: n= 10.78 moles. Corrected Volume=…
Q: Calculate the volume (L) occupied by 2.12 moles of nitric oxide (NO) at 6.54 atm and 76 degrees…
A: Ideal gases are observed as the gas molecules that have no force of attraction/repulsion, the volume…
Q: The experiment showed a final pressure of about 17.2 PSI starting from 15 PSI after heating the…
A:
Q: 10) A is an instrument used to measure atmospheric pressure specifically in the area of meteorology.…
A: Answer Instrument used to measure atmosphere pressure specified…
Q: The characteristics of a certain gas are listed in the table. Characteristics of a Gas…
A: Given information is as follows: Mass of the sample = 3.2 g The STP condition means; T = 273.15 K P…
Q: The mercury on the short side of a J-tube is at 205.0 mm and the long side is at 980.2 mm. What is…
A: Atmospheric water can be calculated using the given Pressure of mercury on both side of j- tube.
Q: You are conducting an experiment using an unknown compound containing carbon, hydrogen, and oxygen.…
A: Given:Mass of unknown compound =7.26gMass of water formed =2.7gVolume of CO2 collected over water…
Q: What are each of the following observations an example of? Drag the appropriate items to their…
A:
Q: Also, answer the following questions in the conclusion section of your lab notebook using complete…
A:
Q: Molecular weight of a volatile liquid A student had the following data: mass of volatile liquid:…
A:
Q: When measuring the flask volume by filling with water, what would happen to the calculated volume of…
A: The space occupied by any substance is called as the volume of the substance. The volume of the…
Q: Let say, the same aluminum cylindrical container (only container not the situation) is fitted with a…
A:
Q: Which gas deviates most from ideal behavior?
A: Since at STP all ideal gases should have 22.41 L of molar volume. Hence the gas which is having…
Q: Very low temperatures can be studied by slowing down atoms in the gas phase using crossed molecular…
A: Root mean square speed of a molecule can be calculated using the equation given below: Vrms = 3RTMM…
Q: A spherical basketball has a diameter of 10.0 inches, and is inflated to a total air pressure of 1.6…
A: Volume of sphere can be calculated using the formula given below. Here, r is the radius of sphere.
Q: The pressure on 2.5 L of hydrogen gas changes from 105 Kpa to 40.5 kPa. What will the new volume be…
A:
Q: Given the data, what pressure would the butane gas sample have at a volume of 135.0ml? Please show…
A: According to Boyle's law, at constant temperature(T) and the number of moles of gas(n), the…
Q: The prevailing atmospheric pressure on a plateau in Colorado is 0.786 atm. Express this pressure in…
A: Given :- pressure = 0.786 atm To calculate :- given pressure in ; psi KPa Pa mm Hg in Hg
Q: Br2 is a liquid with a density of 3.03 g/mL. Determine the pressure in atm exerted by a column…
A: Given Br2 is a liquid with a density of 3.03 g/mL, or 3.03 g/cm3. Hence the pressure exerted by a…
Q: A 335 mL sample of Oxygen gas at 25oC is heated to 50.0oC. If the pressure remains constant, what is…
A: Given, Initial Volume = 335 mL Initial Temperature = 25.0°C Final temperature = 50.0 °C Final…
Q: Enter your answer in the provided box. Solid white phosphorus melts and then vaporizes at high…
A: Rate of effusion of gaseous white Phosphorous = RP Rate of effusion of Neon = RNe It is given that…
Q: Part IV: Relationships Between Gas Variables Scientists in the late 1800’s noted relationships…
A: Variables Constant Parameters Relationship Proportionality (see hint below) pressure,…
Q: For the following set of pressure/volume data, calculate the new volume of the gas sample after the…
A: given data - volume = V1 = 115 ml pressure = P1 = 738 mm Hg volume = V2 = ? pressure = P2 = 781 mm…
Calculate the volume of the flask using the mass of water which filled the flask and the density of water at the measured temperature. In addition to the volume of the flask, you will need to add 3.0 mL to account for the volume of the tube that leads to the pressure sensor.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

- If 35,000 kg of whole milk containing 4% fat is to be separated in a 6-hour period into skim milk with 0.45% fat and cream with 45% fat, what are the mass flow rates of the two output streams from a continuous centrifuge which accomplishes this separation? (Ans; Cream=464.8335kg/h, Skim milk= 5368.4998kg/h)You are absolutely the BEST. Finally someone that dosen't calculate this exercise as 836f66dgsffjgvkik!!!! I understand everything you did, but I have one question. Some experts says that km can be calculated by saying Vmax from date divided by 2 and then they find Km by going down on x-axis on the graph. But, you used the MM equation, to solve for Km. Is both method the same? Or is one of them more precise than the other? *And if theres no need to reply back after you answering my question, then I will thank you again for your help. I have more these types of exam questions that I will probably upload later, and I hope someone like you will look at it.1. Estimate the density of a 25-API gravity dead oil at 100 F.
- Sum of coefficients C7H8 + O2 --> CO2 + H2O after balancingA 120. MW coal plant wants to add a SOXemissions control system to reduce its emissions. The emission control system has a capitalcost of $815 per kW and reduces SOX emissions by 98% to 0.0918 kg/MWh. Assume thelifetime of the emission control system is 20 years and the power plant produces 650GWh/y. a)What is the annualized cost of this system in dollars per year if the discount rate (interestrate) is 7.0 percent/year? What if the discount rate is 4.0 percent/year? b)What is the cost per 1.0399 x 104 kWh of electricity usage (the average annual U.S.residential electricity consumption in 2017 according to the EIA) at each of the discountrates specified in part a? c)What is the cost per metric ton of SOx removed at each of the discount rates specified inpart a? Grammer Chicken produces canned chicken a la king. The chicken a la king passes through three departments: (1) Mixing, (2) Retort (sterilization), and (3) Packing. In the Mixing Department, chicken and cream are added at the beginning of the process, the mixture is partly cooked, and chopped green peppers and mushrooms are added at the end of the process. Conversion costs are incurred evenly throughout the mixing process. November data from the Mixing Department as follows: 1 Summarize the flow of physical units and compute the equivalent units. (Hint: Each direct material added at a different point in the production process requires its own equivalent-unit computation.) i. Check your spelling carefully and do not abbreviate. ii. Follow the format of the exhibit that shows the flow of physical units and output in terms of equivalent units. iii. Complete all input areas. Be sure to include any zero balances in the report. 2 Compute the cost per equivalent unit for…
- Downvoted for wrong solution. A river is carrying water containing 2000 mg/l Magnesium Chloride into a small lake. The lake has a naturally occurring Magnesium Chloride of 50 mg/l. If the river flow is 2500 Lmin and the lake flow rate is 1.5 m³.sec¹, what is the concentration of MgCl2 in the lake after the discharge point? Assume that the flows in the river and lake are completely mixed, that the salt is a conservative substance, and the system is at steady state.Estimate the Km and vmax from the data. [S] (M) Velocity (µM/min) 2.5 x 10-6 28 .00001 70 .00004 112 .0001 128 .002 139 .01 140 Km=.00001 vmax=140 Km=.002 vmax=112 Km=.01 vmax=140 Km=.00001 vmax=70Calculate the specific volume, α (cm3/gm), using the following equation and the values of constants and conversion factors provided. α = (RT)/p Where R = 2.8704 x 106 erg/gm(˚K), T = 10˚C, and p = 1000 millimars (mb). All of the conversion factors you will need are: 1 mb = 103 dynes/cm2 ˚K = 273˚ + ˚C erg = dyne(cm) a. α = 8.123 x 109 cm3/gm b. α = 8.123 x 102 cm3/gm c. α = 2.8704 x 104 cm3/gm d. α = 2.8704 x 106 cm3/gm
- Cereal is being dried in a vertical drier by air flowing countercurrent to the cereal. To prevent breakage of the cereal flakes, some of the exit air from the drier is recycled back to mix with the the moist fresh air. For each 1000 kg/hr of wet cereal fed to the drier, calculate the input of moist fresh air in kg/hr and recycle rate in kg/hr. The available data on stream compositions is (fractions): For water: Fresh air: 0.0132 Wet cereal: 0.200 Exit air: 0.263 Dried Cereal: 0.050 Air entering Dryer: 0.066Medicare does not cover the cost of this prescription medicine, which currently averages $297.78 per kilogram. The theoretical yield for the pure barium sulfate you were transferring was 500.00kg. You spilled 27.45 kg. What is the % yield of the transfer that you need to report? And also determine the consumer price of the spilled barium sulfate.Please answer fast it’s very important and urgent I say very urgent so please answer super super fast please For the image attached For 1. a Mass of metal: Trial 1 is 35.0228 g Trial 2 is 35.0915 g Trial 3 is 34.0821 g Mass of water: Trial 1 is 20.0177 g Trial 2 is 20.0250 g Trial 3 is 20.0168 g For delta t of water: Trial 1 is 15.5 C Trial 2 is 15.7 C Trial 3 is 15.1 C For delta t of metal Trial 1 is 80.1 C Trial 2 is 80.2 C Trial 3 is 79.5 C For B my calculated Specific heat is: Trial 1 is 0.462 Trial 2 is 0.467 Trial 3 is 0.466

