Calculate u of 1 mole of helium gas at the top of a mountain (7000 m) under the assumption that the air is just He gas with p = 1 atm at 0 m and T = 300 K from 0 m to 7000 m. Calculate your answer in eV, but do not include units in your answer. Write your answer in normal form with 2 decimal places (such as X.XX or XX.XX, etc.).
Calculate u of 1 mole of helium gas at the top of a mountain (7000 m) under the assumption that the air is just He gas with p = 1 atm at 0 m and T = 300 K from 0 m to 7000 m. Calculate your answer in eV, but do not include units in your answer. Write your answer in normal form with 2 decimal places (such as X.XX or XX.XX, etc.).
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 48E: Although the preferred SI unit of area is the square meter, land is often measured in the metric...
Related questions
Question
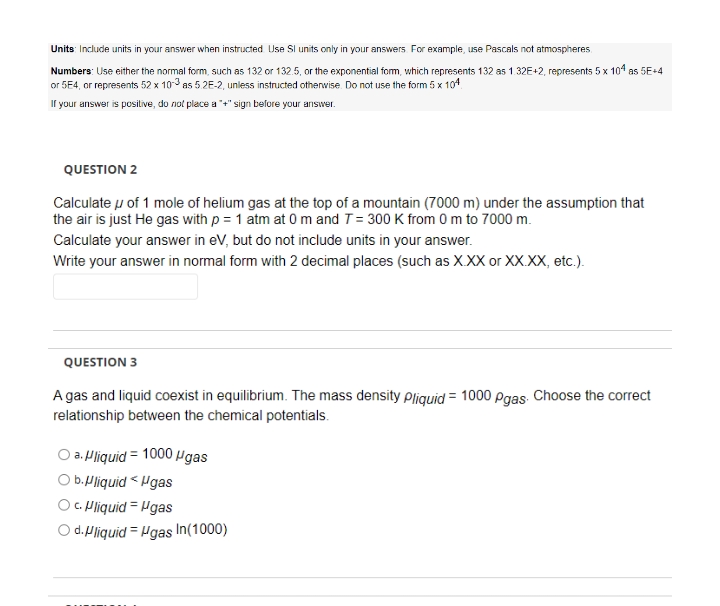
Transcribed Image Text:Units: Include units in your answer when instructed Use SI units only in your answers For example, use Pascals not atmospheres.
Numbers: Use either the normal form, such as 132 or 132.5, or the exponential form, which represents 132 as 1.32E+2, represents 5 x 104 as 5E+4
or 5E4, or represents 52 x 10-3 as 5 2E-2, unless instructed otherwise. Do not use the form 5 x 104
If your answer is positive, do not place a "+" sign before your answer.
QUESTION 2
Calculate u of 1 mole of helium gas at the top of a mountain (7000 m) under the assumption that
the air is just He gas with p = 1 atm at 0m and T= 300 K from 0 m to 7000 m.
Calculate your answer in eV, but do not include units in your answer.
Write your answer in normal form with 2 decimal places (such as X.XX or XX.XX, etc.).
QUESTION 3
A gas and liquid coexist in equilibrium. The mass density pliquid = 1000 pgas Choose the correct
relationship between the chemical potentials.
a. Pliquid = 1000 µgas
O b.Mliquid < Hgas
C. Pliquid = Hgas
d.Pliquid = Hgas In(1000)
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning