Calculating entropy change using the Boltzmann hypothesis Suppose additional surface becomes exposed, so that 64 adsorption sites are now available for the molecule. Calculate the change in entropy. One way an 0, molecule could be adsorbed on a patch of An oxygen (0, molecule is adsorbed on a patch of surface (see sketch at right). This patch is known to contain 36 adsorption surface with l16 sites. sites. The (0, molecule has enough energy to move from site to site, so it could be on any one of them. Round your answer to 3 significant digits, and be sure it has the correct unit symbol. x10 X www.sMwy www.ew www.wwwae ww.watwdhwwwlaww wwwwwwiwwwewaiw wwwwinanil.comeww www.wwhwtewawaiwww AWAw.wwhwwmiwiwwweaw.cm ww.wiasia www.w.mmkingl.use Privacy Terms of Use 2019 McGraw-Hill Education. All Rights Reserved. Check Explanation
Calculating entropy change using the Boltzmann hypothesis Suppose additional surface becomes exposed, so that 64 adsorption sites are now available for the molecule. Calculate the change in entropy. One way an 0, molecule could be adsorbed on a patch of An oxygen (0, molecule is adsorbed on a patch of surface (see sketch at right). This patch is known to contain 36 adsorption surface with l16 sites. sites. The (0, molecule has enough energy to move from site to site, so it could be on any one of them. Round your answer to 3 significant digits, and be sure it has the correct unit symbol. x10 X www.sMwy www.ew www.wwwae ww.watwdhwwwlaww wwwwwwiwwwewaiw wwwwinanil.comeww www.wwhwtewawaiwww AWAw.wwhwwmiwiwwweaw.cm ww.wiasia www.w.mmkingl.use Privacy Terms of Use 2019 McGraw-Hill Education. All Rights Reserved. Check Explanation
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter17: Statistical Thermodynamics: Introduction
Section: Chapter Questions
Problem 17.14E
Related questions
Question
Calculate the change in entropy round to three sig figs. Answer should be J/k and be around ten to the -24 power or around there. Please check your math and don't round during the math. Only for answer
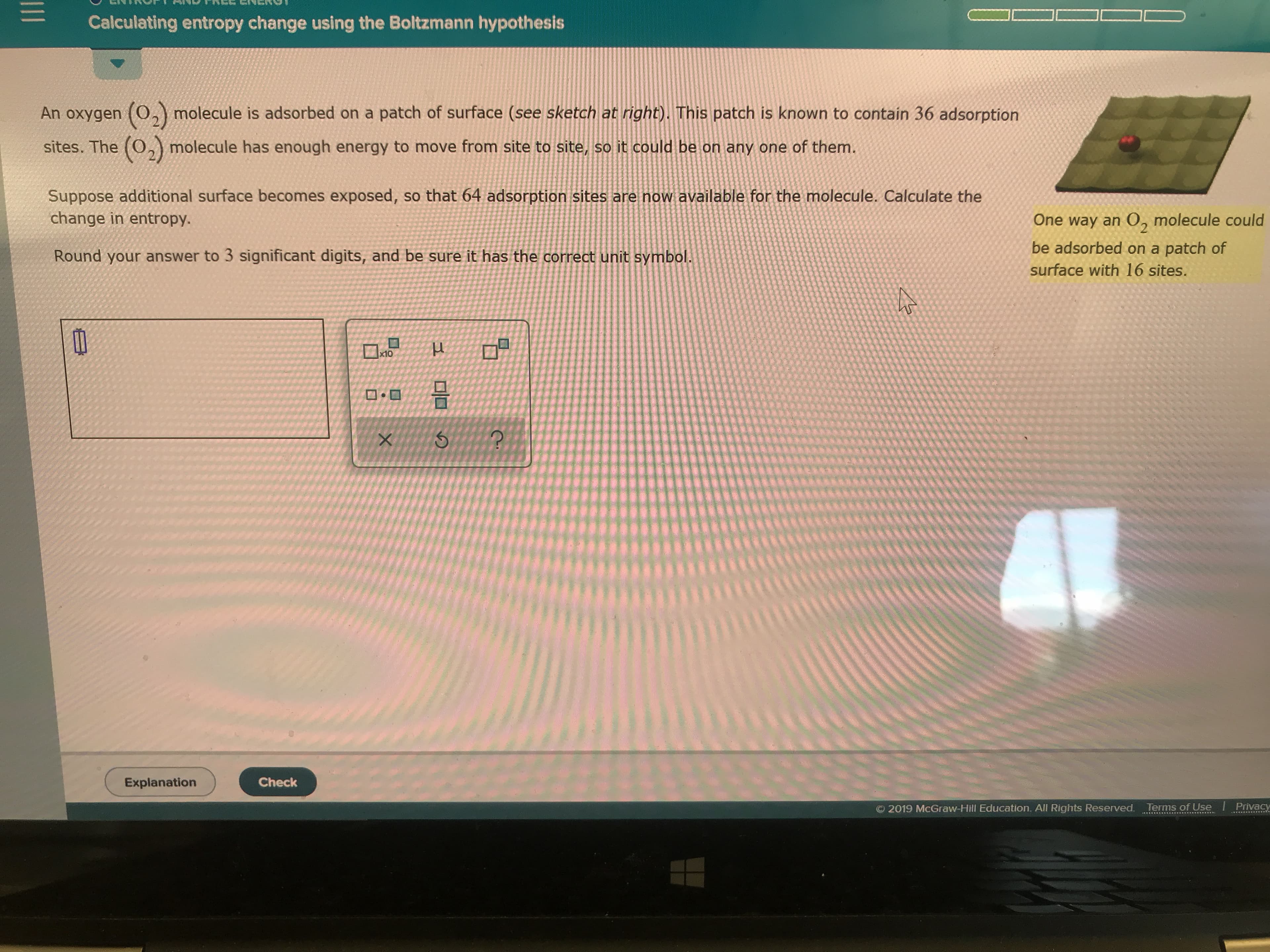
Transcribed Image Text:Calculating entropy change using the Boltzmann hypothesis
Suppose additional surface becomes exposed, so that 64 adsorption sites are now available for the molecule. Calculate the
change in entropy.
One way an 0, molecule could
be adsorbed on a patch of
An oxygen (0, molecule is adsorbed on a patch of surface (see sketch at right). This patch is known to contain 36 adsorption
surface with l16 sites.
sites. The (0, molecule has enough energy to move from site to site, so it could be on any one of them.
Round your answer to 3 significant digits, and be sure it has the correct unit symbol.
x10
X
www.sMwy
www.ew
www.wwwae
ww.watwdhwwwlaww
wwwwwwiwwwewaiw
wwwwinanil.comeww
www.wwhwtewawaiwww
AWAw.wwhwwmiwiwwweaw.cm
ww.wiasia
www.w.mmkingl.use
Privacy
Terms of Use
2019 McGraw-Hill Education. All Rights Reserved.
Check
Explanation
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning