Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter6: Electronic Structure And The Periodic Table
Section: Chapter Questions
Problem 51QAP: How many unpaired electrons are there 111 the following ions? (a) V3+(b) Sn4+(c) I-(d) W4+
Related questions
Question
Can you create a Atom Model of Oxygen using the Aufbau’s Principle and Hund’s Rule of Maximum Multiplicity.
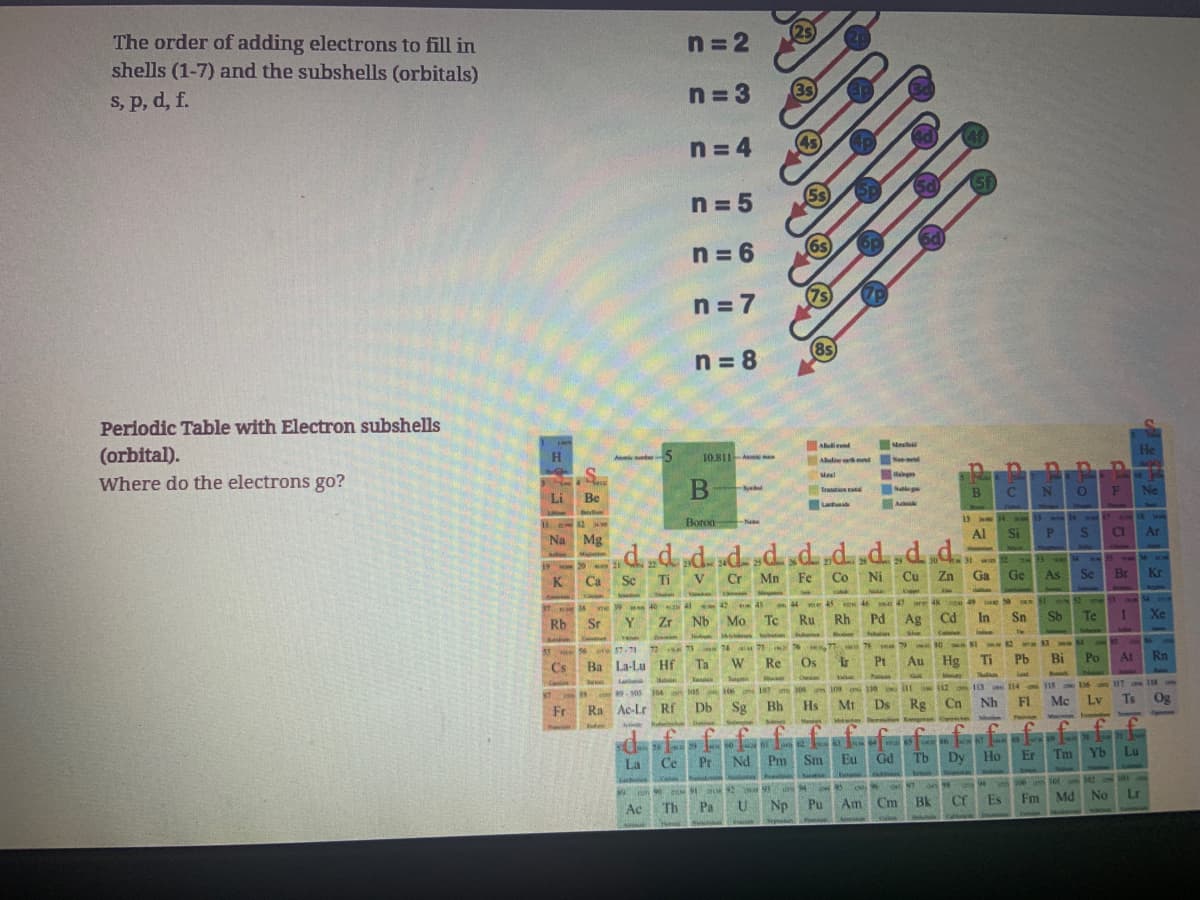
Transcribed Image Text:The order of adding electrons to fill in
shells (1-7) and the subshells (orbitals)
s, p, d, f.
Periodic Table with Electron subshells
(orbital).
Where do the electrons go?
H
Li
S
Be
By
Rb
MON 20
20 way 21
K Ca Sc
Cs
18
Fr
Sr
-5
Y
HOM
17.71
Ba La-Lu Hf
Ti
La
40
Ac
Zr
n=2
n=3
n=4
n=5
n=6
n=7
n=8
B
Boron Ne
10.811-
71
V
Ta
559
Ce
Na Mg d.d.d.d.d.d.d.d.d.d..
Cr Mn Fe
-Spl
100 104
Ra Ac-Lr Rf Db Sg
E D
(62) 76
74 75
Re
W
43
Nb Mo Tc Ru
S
£ f
10
Pr
(35)
S
106 107 108
Bh
61
45
(5s
90 91 92 93
Th
U
Pa
Np
6s
7s
(8s)
Aholi und
Mesid
Aluline
EE
Meal
Halogen
Tranation extal
Sulle pa
Os
12
Lash
Hs
Handys
Nd Pm Sm
944
Pu
Co
4p
Rh
op
Mt
Min
Ni
Ad
Pt
Cu
Pd Ag
Au
11011
111
Ds
Zn
IN
Cd
Comer
4f
47 re 48 49
on
Am Cm Bk
B
13 14 15
Si
Al
31
47 on 98
5f
65 6
&
Eu
A
Gd
Tb Dy
Ga
Cn
Rg
Cop
In
in
81
Cr
Catern
29 50 51
Sn
Hg
Ti
Pb
My Thatsun
Land
112
111 on 113 114
CN
Ge
67
Ho
Es
P
Er
Sb
13
FI
Nh
Revie
Bi
Bond
115
33 34 JAN 35-36
As
Se
O
16
Mc
Main
19
S Cl
52
F
Po
Te 1
Tm Yb
101
Fm Md No
116 117
Lv
Br Kr
He
Tre
21
Ne
At Rn
2
Ar
Lu
Ts Og
Lr
WHEN
Xe
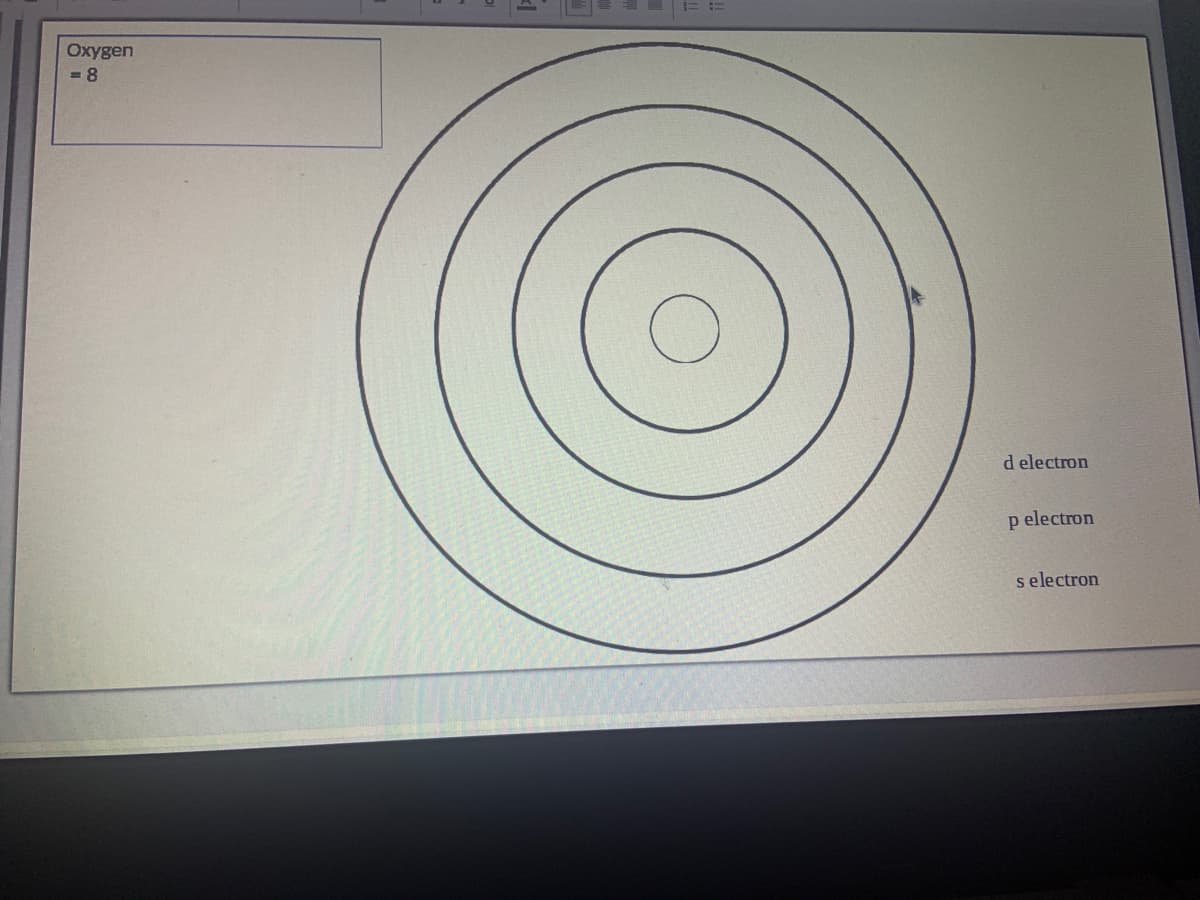
Transcribed Image Text:Oxygen
= 8
d electron
p electron
s electron
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning