Carbon monoxide gas reacts with hydrogen gas to form methanol via the following reaction: CO(g)2H2 (g)->CH3 OH(g) Part A A 1.50 L reaction vessel, initially at 305 K, contains carbon monoxide gas at a partial pressure of 232 mmHg and hydrogen gas at a partial pressure of 367 mmHg Determine the theoretical yield of methanol in grams. Express your answer with the appropriate units. ? You may want to reference (Pages 444 - 446) Section 10.10 while completing this problem Value Units Request Answer Submit
Carbon monoxide gas reacts with hydrogen gas to form methanol via the following reaction: CO(g)2H2 (g)->CH3 OH(g) Part A A 1.50 L reaction vessel, initially at 305 K, contains carbon monoxide gas at a partial pressure of 232 mmHg and hydrogen gas at a partial pressure of 367 mmHg Determine the theoretical yield of methanol in grams. Express your answer with the appropriate units. ? You may want to reference (Pages 444 - 446) Section 10.10 while completing this problem Value Units Request Answer Submit
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 97E: Hydrogen azide, HN3, decomposes on heating by the following unbalanced equation: HN3O(g)N2(g)+H2(g)...
Related questions
Question
100%
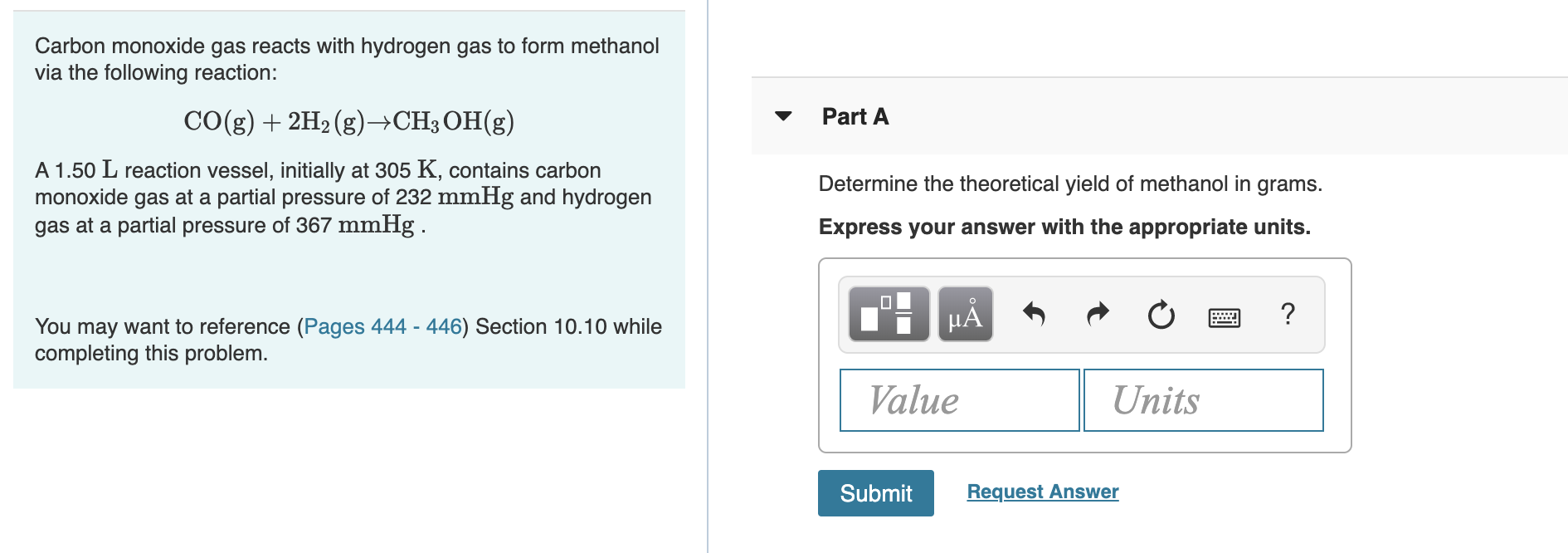
Transcribed Image Text:Carbon monoxide gas reacts with hydrogen gas to form methanol
via the following reaction:
CO(g)2H2 (g)->CH3 OH(g)
Part A
A 1.50 L reaction vessel, initially at 305 K, contains carbon
monoxide gas at a partial pressure of 232 mmHg and hydrogen
gas at a partial pressure of 367 mmHg
Determine the theoretical yield of methanol in grams.
Express your answer with the appropriate units.
?
You may want to reference (Pages 444 - 446) Section 10.10 while
completing this problem
Value
Units
Request Answer
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning