CH 09 HW xercise 9.21 Part A Given the balanced equation, calculate the mass of product that can be prepared from 2.10 g of zinc metal. 2Zn(s)+02(g)2 ZnO (s) S Express your answer with the appropriate units. ? Value g mzno = Previous Answers Request Answer Submit X Incorrect; Try Again; 4 attempts remaining Provide Feedback Return to Assianment P Pearson MacBook Air DII 000 O00 F 9 F8 F7 F6 F5 F4 F3 F2 & $ 4 5 U Y W E CO < CO T LO ** 3
CH 09 HW xercise 9.21 Part A Given the balanced equation, calculate the mass of product that can be prepared from 2.10 g of zinc metal. 2Zn(s)+02(g)2 ZnO (s) S Express your answer with the appropriate units. ? Value g mzno = Previous Answers Request Answer Submit X Incorrect; Try Again; 4 attempts remaining Provide Feedback Return to Assianment P Pearson MacBook Air DII 000 O00 F 9 F8 F7 F6 F5 F4 F3 F2 & $ 4 5 U Y W E CO < CO T LO ** 3
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.100QE
Related questions
Question
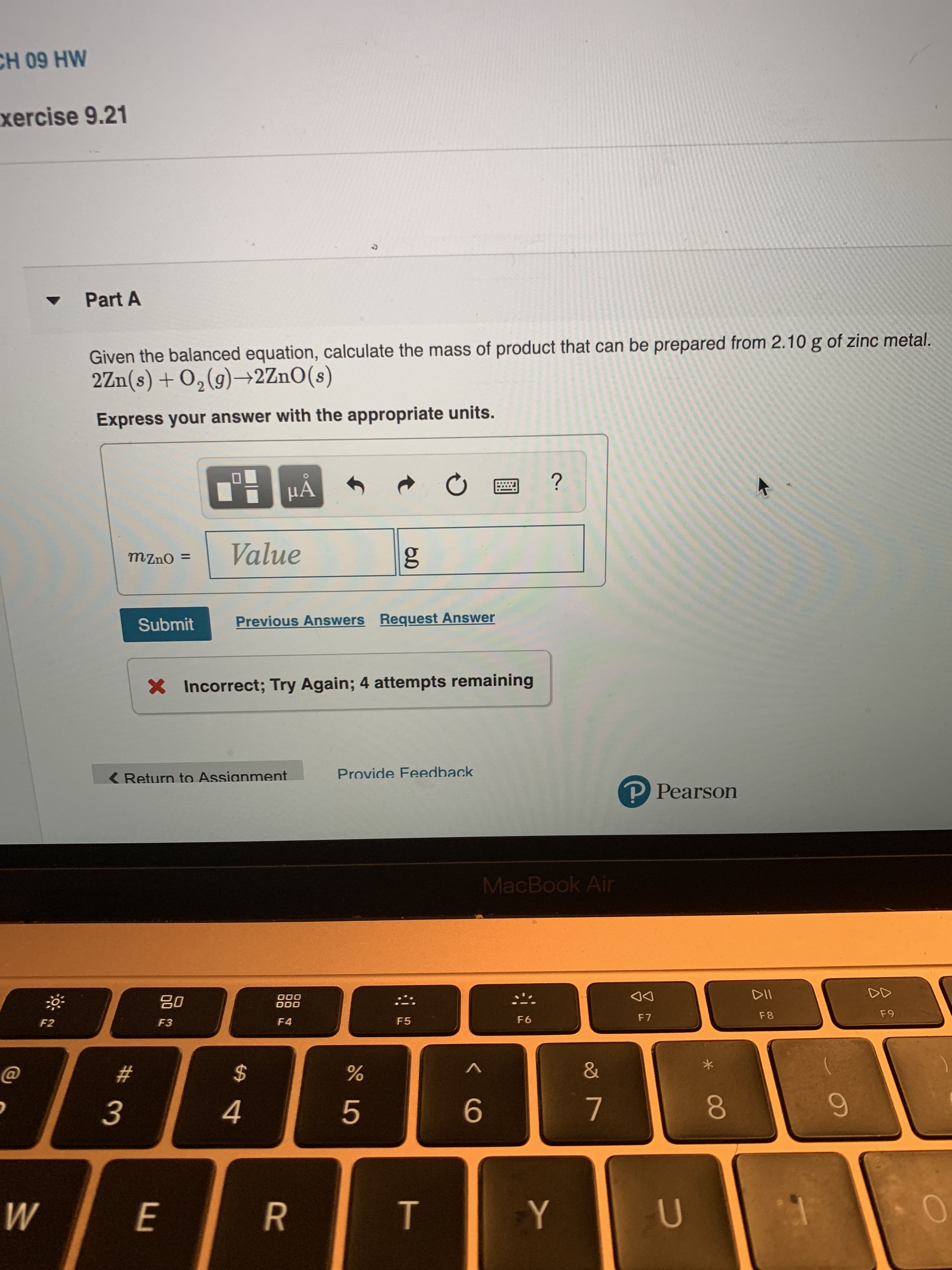
Transcribed Image Text:CH 09 HW
xercise 9.21
Part A
Given the balanced equation, calculate the mass of product that can be prepared from 2.10 g of zinc metal.
2Zn(s)+02(g)2 ZnO (s)
S
Express your answer with the appropriate units.
?
Value
g
mzno =
Previous Answers Request Answer
Submit
X Incorrect; Try Again; 4 attempts remaining
Provide Feedback
Return to Assianment
P Pearson
MacBook Air
DII
000
O00
F 9
F8
F7
F6
F5
F4
F3
F2
&
$
4
5
U
Y
W
E
CO
< CO
T
LO
** 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning