Characterize each of the following pure compounds according to their dominant intermolecular force London dispersion ion-dipole No Answers Chosen No Answers Chosen Hydrogen bonds dipole-dipole No Answers Chosen No Answers Chosen Possible answers E CH3CH2CH=CHCH3 ! CH3CH2CH2OH E CH3CH2CH2CHO E CH3CH2COOH
Characterize each of the following pure compounds according to their dominant intermolecular force London dispersion ion-dipole No Answers Chosen No Answers Chosen Hydrogen bonds dipole-dipole No Answers Chosen No Answers Chosen Possible answers E CH3CH2CH=CHCH3 ! CH3CH2CH2OH E CH3CH2CH2CHO E CH3CH2COOH
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 18E: Under certain conditions, molecules of acetic acid, CH3COOH, form dimers, pairs of acetic acid...
Related questions
Question
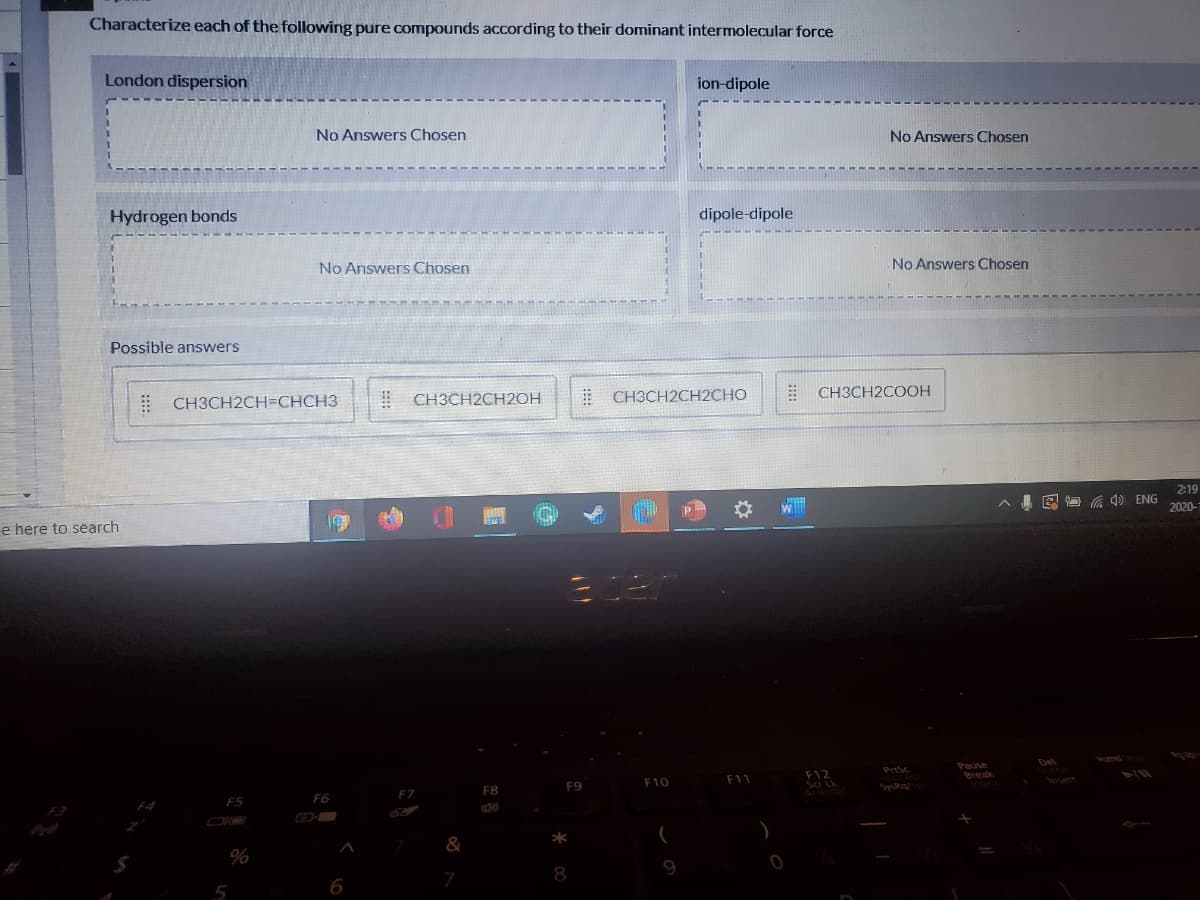
Transcribed Image Text:Characterize each of the following pure compounds according to their dominant intermolecular force
London dispersion
ion-dipole
No Answers Chosen
No Answers Chosen
Hydrogen bonds
dipole-dipole
No Answers Chosen
No Answers Chosen
Possible answers
| CH3CH2CH=CHCH3
! CH3CH2CH2OH
| CH3CH2CH2CHO
E CH3CH2COOH
2:19
O E O 0 ENG
2020-
P
e here to search
Del
Pause
Break
PrSc
F12
Scr Lk
F10
F11
insen
F8
F9
SysRa
F6
F7
F5
&
%
7
8
6
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax