Charged Particle Concentration (mM) Inside Concentration (mM) Outside Na 15 150 K- 150 A- 100 Based on the total concentration of charge on each side of the cell, the outside of the cell has positive charge than the inside of the cell. Remember that ions are electrically attracted to particles of opposite charge and are repelled by particles of the same charge. By electrical attraction alone, in what direction would K- tend to move if movement were possible? By diffusion alone, in what direction would K- ions tend to move if movement were possible? If a gated K- channel in the membrane opened up, in what direction would K move? O Depends on the relative strengths of diffusion and electrical attraction O Into the cell O Out of the cell The tendency of K ions to move across the plasma membrane depends on the
Charged Particle Concentration (mM) Inside Concentration (mM) Outside Na 15 150 K- 150 A- 100 Based on the total concentration of charge on each side of the cell, the outside of the cell has positive charge than the inside of the cell. Remember that ions are electrically attracted to particles of opposite charge and are repelled by particles of the same charge. By electrical attraction alone, in what direction would K- tend to move if movement were possible? By diffusion alone, in what direction would K- ions tend to move if movement were possible? If a gated K- channel in the membrane opened up, in what direction would K move? O Depends on the relative strengths of diffusion and electrical attraction O Into the cell O Out of the cell The tendency of K ions to move across the plasma membrane depends on the
Human Physiology: From Cells to Systems (MindTap Course List)
9th Edition
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Lauralee Sherwood
Chapter3: The Plasma Membrane And Membrane Potential
Section: Chapter Questions
Problem 7UC: Describe the contribution of each of the following to establishing and maintaining membrane...
Related questions
Question
How do you do this?
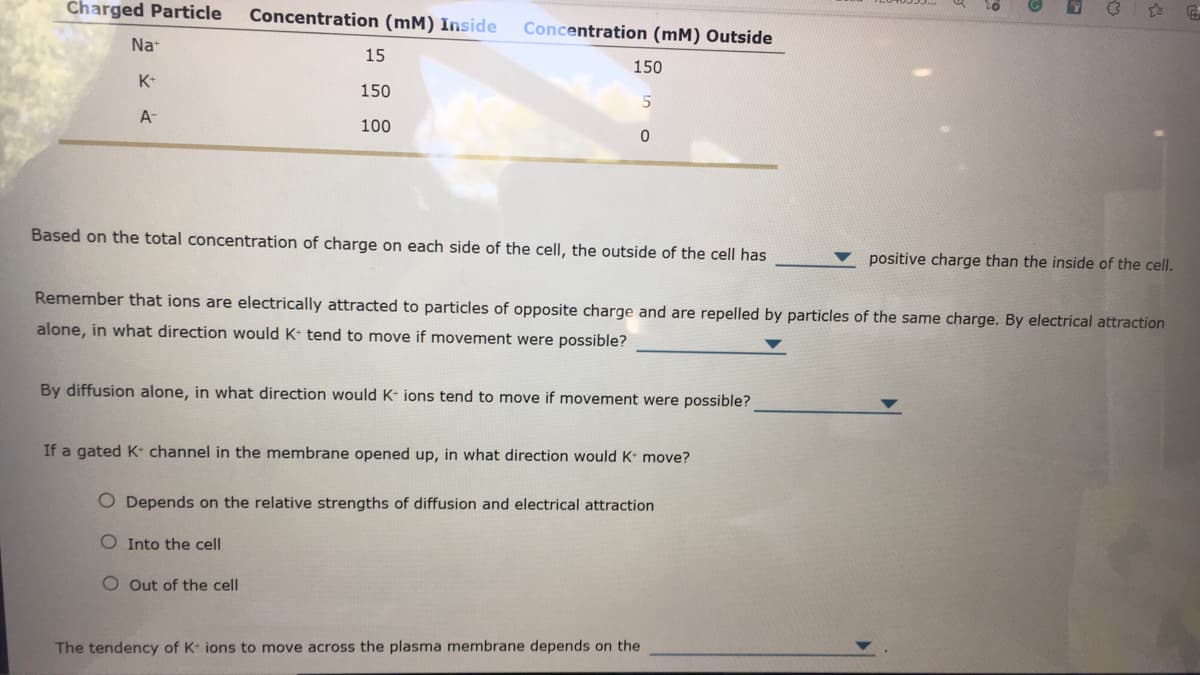
Transcribed Image Text:Charged Particle
Concentration (mM) Inside
Concentration (mM) Outside
Na
15
150
K-
150
A-
100
Based on the total concentration of charge on each side of the cell, the outside of the cell has
positive charge than the inside of the cell.
Remember that ions are electrically attracted to particles of opposite charge and are repelled by particles of the same charge. By electrical attraction
alone, in what direction would K tend to move if movement were possible?
By diffusion alone, in what direction would K- ions tend to move if movement were possible?
If a gated K- channel in the membrane opened up, in what direction would K move?
O Depends on the relative strengths of diffusion and electrical attraction
O Into the cell
O Out of the cell
The tendency of K ions to move across the plasma membrane depends on the
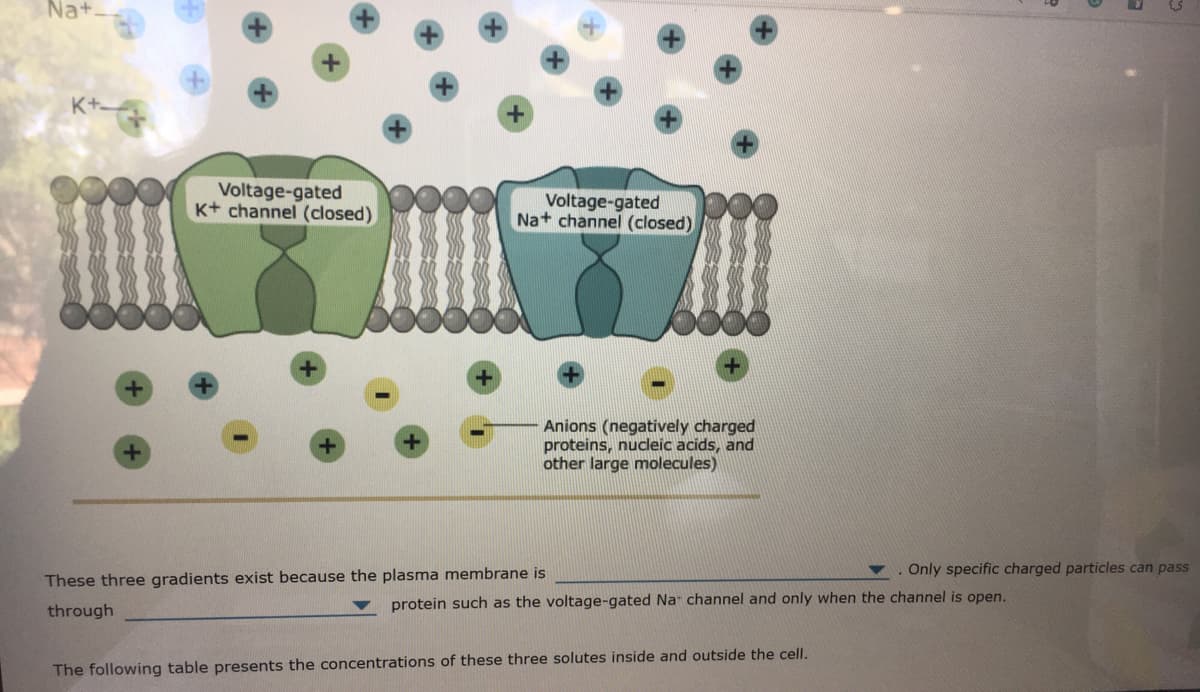
Transcribed Image Text:Na+
Voltage-gated
K+ channel (closed)
Voltage-gated
Na+ channel (closed)
Anions (negatively charged
proteins, nuceic acids, and
other large molecules)
Only specific charged particles can pass
These three gradients exist because the plasma membrane is
through
protein such as the voltage-gated Na channel and only when the channel is open.
The following table presents the concentrations of these three solutes inside and outside the cell.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning

Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning