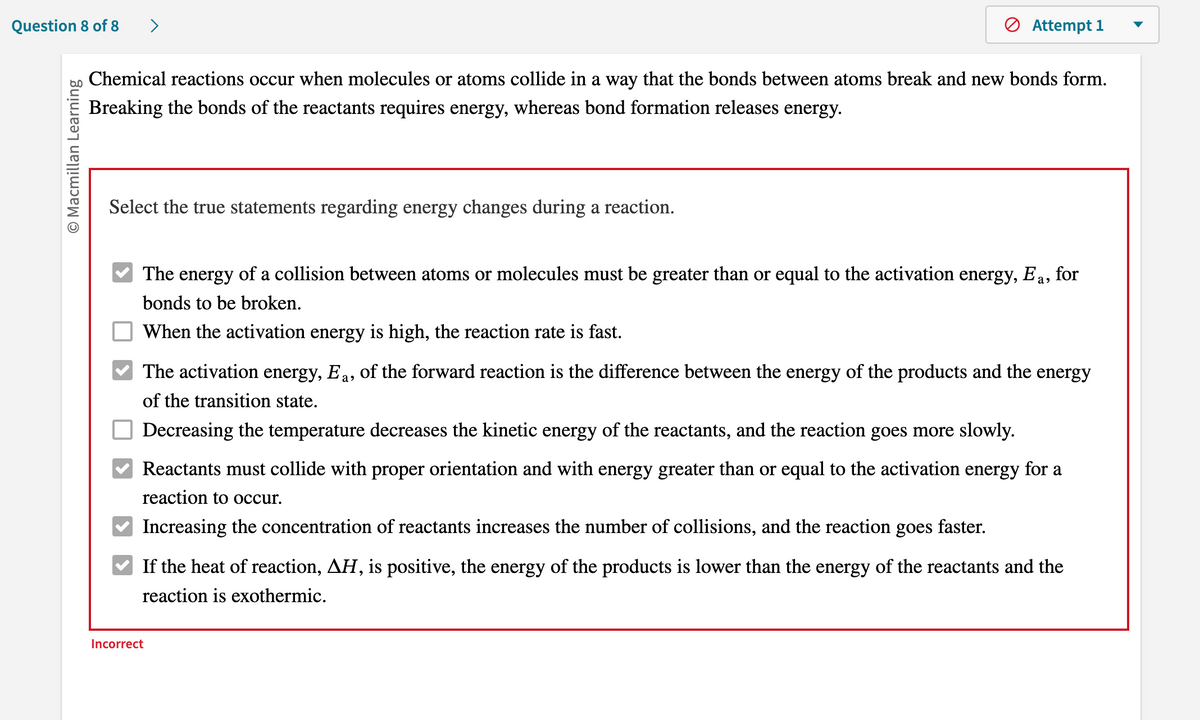
Chemical reactions occur when molecules or atoms collide in a way that the bonds between atoms break and new bonds form. Breaking the bonds of the reactants requires energy, whereas bond formation releases energy. Select the true statements regarding energy changes during a reaction. The energy of a collision between atoms or molecules must be greater than or equal to the activation energy, Ea, for bonds to be broken. When the activation energy is high, the reaction rate is fast. The activation energy, Ea, of the forward reaction is the difference between the energy of the products and the energy of the transition state. Decreasing the temperature decreases the kinetic energy of the reactants, and the reaction goes more slowly. Reactants must collide with proper orientation and with energy greater than or equal to the activation energy for a reaction to occur. Increasing the concentration of reactants increases the number of collisions, and the reaction goes faster. If the heat of reaction, AH, is positive, the energy of the products is lower than the energy of the reactants and the reaction is exothermic.
Chemical reactions occur when molecules or atoms collide in a way that the bonds between atoms break and new bonds form. Breaking the bonds of the reactants requires energy, whereas bond formation releases energy. Select the true statements regarding energy changes during a reaction. The energy of a collision between atoms or molecules must be greater than or equal to the activation energy, Ea, for bonds to be broken. When the activation energy is high, the reaction rate is fast. The activation energy, Ea, of the forward reaction is the difference between the energy of the products and the energy of the transition state. Decreasing the temperature decreases the kinetic energy of the reactants, and the reaction goes more slowly. Reactants must collide with proper orientation and with energy greater than or equal to the activation energy for a reaction to occur. Increasing the concentration of reactants increases the number of collisions, and the reaction goes faster. If the heat of reaction, AH, is positive, the energy of the products is lower than the energy of the reactants and the reaction is exothermic.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 10CR: . Explain what it means that a reaction has reached a state of chemical equilibrium. Explain why...
Related questions
Question
Transcribed Image Text:Question 8 of 8
O Macmillan Learning
>
Chemical reactions occur when molecules or atoms collide in a way that the bonds between atoms break and new bonds form.
Breaking the bonds of the reactants requires energy, whereas bond formation releases energy.
Select the true statements regarding energy changes during a reaction.
Attempt 1
The energy of a collision between atoms or molecules must be greater than or equal to the activation energy, Ea, for
bonds to be broken.
When the activation energy is high, the reaction rate is fast.
The activation energy, Ea, of the forward reaction is the difference between the energy of the products and the energy
of the transition state.
Decreasing the temperature decreases the kinetic energy of the reactants, and the reaction goes more slowly.
Reactants must collide with proper orientation and with energy greater than or equal to the activation energy for a
reaction to occur.
Increasing the concentration of reactants increases the number of collisions, and the reaction goes
Incorrect
faster.
If the heat of reaction, AH, is positive, the energy of the products is lower than the energy of the reactants and the
reaction is exothermic.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co