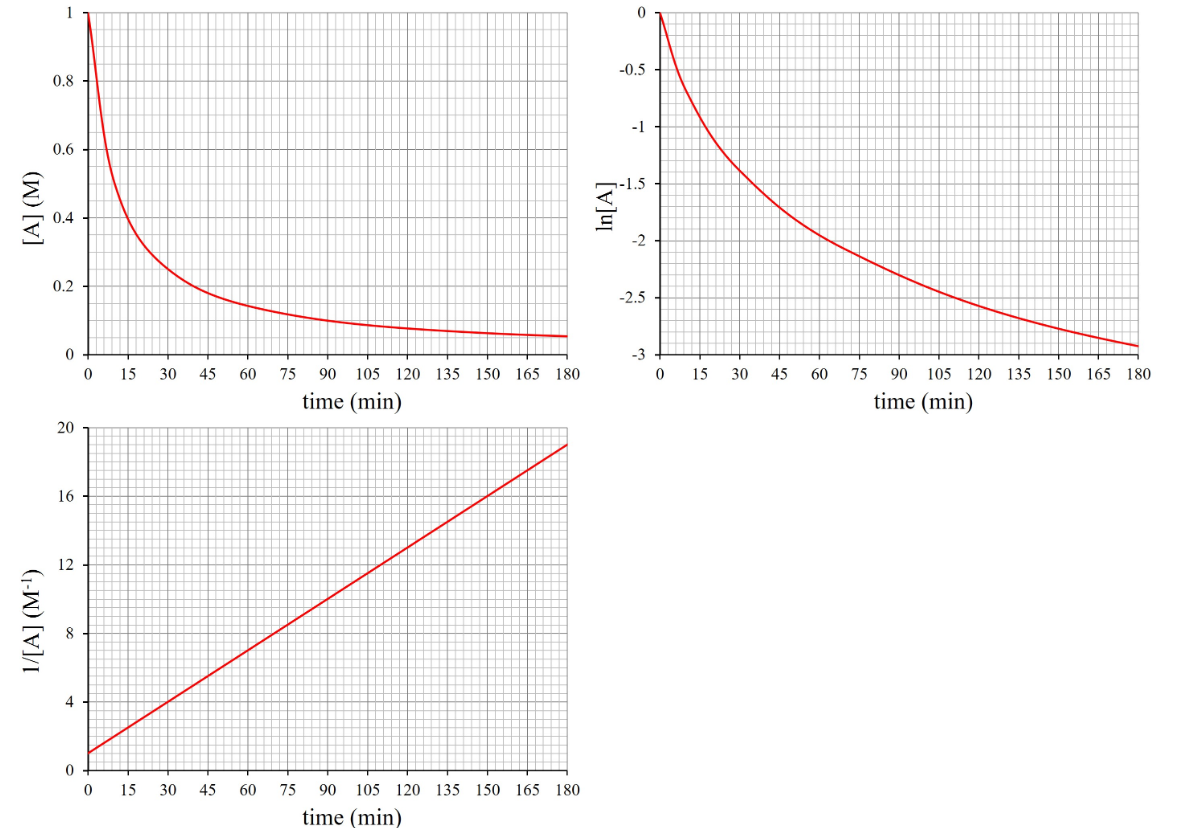
A chemist working for a super glue company is asked to find a way to speed up a 3 component reaction used in the manufacturing process. The general reaction can be written as aA + bB + cC → dD where product D is the super glue. The chemist performs the reaction and measures the concentration of chemical A as time passes. Plotting the obtained data produces the following graphs: If the initial concentration of A is 1.0 M, what is the value of k? *Use correct units and write exponents as unit^-# if necessary.
A chemist working for a super glue company is asked to find a way to speed up a 3 component reaction used in the manufacturing process. The general reaction can be written as aA + bB + cC → dD where product D is the super glue. The chemist performs the reaction and measures the concentration of chemical A as time passes. Plotting the obtained data produces the following graphs: If the initial concentration of A is 1.0 M, what is the value of k? *Use correct units and write exponents as unit^-# if necessary.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 2ALQ: The boxes shown below represent a set of initial conditions for the reaction: Draw a quantitative...
Related questions
Question
A chemist working for a super glue company is asked to find a way to speed up a 3 component reaction used in the manufacturing process. The general reaction can be written as aA + bB + cC → dD where product D is the super glue. The chemist performs the reaction and measures the concentration of chemical A as time passes. Plotting the obtained data produces the following graphs:
If the initial concentration of A is 1.0 M, what is the value of k? *Use correct units and write exponents as unit^-# if necessary.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning