8. Percentage of Silver in silver chloride (Experimental) AgCl 0-6549 100 1.634 мум Ад им ск 9. Percentage of st Agcl (theoretical) MW) Ag C1 = 143-321 = 107.868 = 35.453 10. feccent erгог = 40.33% 75.26 107.868 100 = 75.261 143.321 75.26 % experimental Data Table: Note: All measured masses are to be taken to the third decimal place. Part I: Silver Chloride Mass of empty evaporating dish Mass of silver and evaporating dish before reaction 1. Mass silver 11. 2. Moles of silver Mass silver chloride and evaporating dish after first heating 3. Mass of silver chloride after first heating Mass of silver chloride and evaporating dish after 2nd heating 4. Mass of silver chloride after 2nd heating Mass of silver chloride and evaporating dish after 3rd heating (if necessary) 5. Mass of silver chloride after 3rd heating (if necessary) 6. Mass of chloride 7. Moles of chloride 8. Empirical Formula (experimental) 9. Percentage of silver in silver chloride (experimental) 10. Percentage of silver in silver chloride (theoretical) 11. Percent Error Calculations: = 0.9759 6.88.187-882467 = 0.659 1.88.1879-8+.2129 2. 0.975 gAg. Imolag 3. 87.2129-8818445 87.212-88.84.6. 107.837g/4y = 1.632g = 1.634g -0.009 mol Ag 8. 0.6sager. Imolag 35.4539 = 0.019 mol 87.212 88.187 9. g 0.975 8 0.009 mol 88.844 8 1.632 B 88.846 8 g 1.6348 N/A N/A S g g 10. 6.0 5. 3W.KIND g g mol 0.019 Agclz 40.33% % % %
8. Percentage of Silver in silver chloride (Experimental) AgCl 0-6549 100 1.634 мум Ад им ск 9. Percentage of st Agcl (theoretical) MW) Ag C1 = 143-321 = 107.868 = 35.453 10. feccent erгог = 40.33% 75.26 107.868 100 = 75.261 143.321 75.26 % experimental Data Table: Note: All measured masses are to be taken to the third decimal place. Part I: Silver Chloride Mass of empty evaporating dish Mass of silver and evaporating dish before reaction 1. Mass silver 11. 2. Moles of silver Mass silver chloride and evaporating dish after first heating 3. Mass of silver chloride after first heating Mass of silver chloride and evaporating dish after 2nd heating 4. Mass of silver chloride after 2nd heating Mass of silver chloride and evaporating dish after 3rd heating (if necessary) 5. Mass of silver chloride after 3rd heating (if necessary) 6. Mass of chloride 7. Moles of chloride 8. Empirical Formula (experimental) 9. Percentage of silver in silver chloride (experimental) 10. Percentage of silver in silver chloride (theoretical) 11. Percent Error Calculations: = 0.9759 6.88.187-882467 = 0.659 1.88.1879-8+.2129 2. 0.975 gAg. Imolag 3. 87.2129-8818445 87.212-88.84.6. 107.837g/4y = 1.632g = 1.634g -0.009 mol Ag 8. 0.6sager. Imolag 35.4539 = 0.019 mol 87.212 88.187 9. g 0.975 8 0.009 mol 88.844 8 1.632 B 88.846 8 g 1.6348 N/A N/A S g g 10. 6.0 5. 3W.KIND g g mol 0.019 Agclz 40.33% % % %
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter10: Quantity Relationships In Chemical Reactions
Section: Chapter Questions
Problem 84E
Related questions
Question
Can you please solve #9,10,11 show all of your steps and explain how you do it, the second picture is my work I was trying to do but I don't know if my work is right. SHOW ALL YOUR WORK IN PICTURES! DO NOT TYPE IT.
THank you !

Transcribed Image Text:8. Percentage of Silver in silver chloride (Experimental)
AgCl
0-6549 100
1.634
мум Ад
им ск
9. Percentage of st Agcl (theoretical)
MW) Ag C1 = 143-321
= 107.868
= 35.453
10. feccent erгог
=
40.33%
75.26
107.868 100 = 75.261
143.321
75.26 % experimental
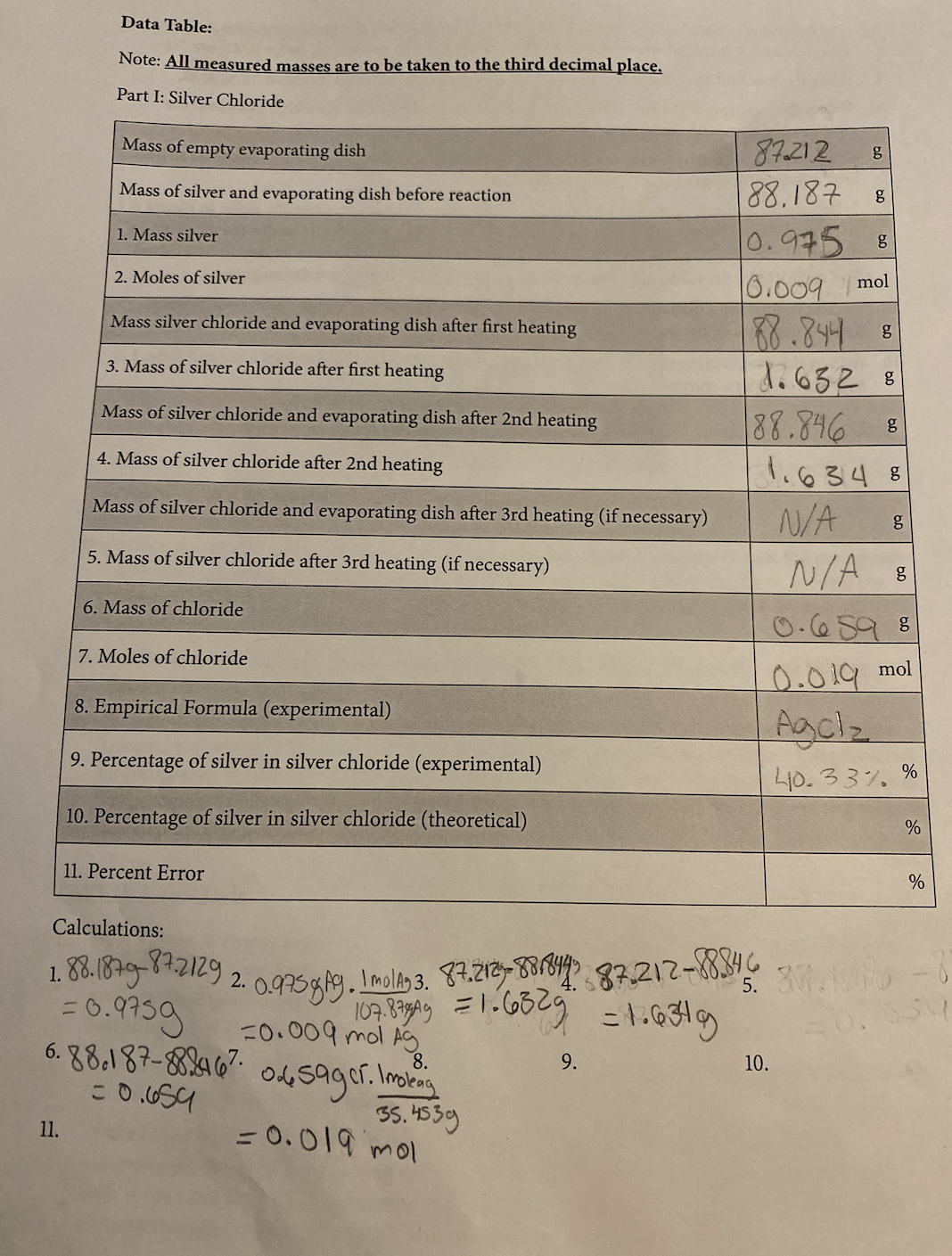
Transcribed Image Text:Data Table:
Note: All measured masses are to be taken to the third decimal place.
Part I: Silver Chloride
Mass of empty evaporating dish
Mass of silver and evaporating dish before reaction
1. Mass silver
11.
2. Moles of silver
Mass silver chloride and evaporating dish after first heating
3. Mass of silver chloride after first heating
Mass of silver chloride and evaporating dish after 2nd heating
4. Mass of silver chloride after 2nd heating
Mass of silver chloride and evaporating dish after 3rd heating (if necessary)
5. Mass of silver chloride after 3rd heating (if necessary)
6. Mass of chloride
7. Moles of chloride
8. Empirical Formula (experimental)
9. Percentage of silver in silver chloride (experimental)
10. Percentage of silver in silver chloride (theoretical)
11. Percent Error
Calculations:
= 0.9759
6.88.187-882467
= 0.659
1.88.1879-8+.2129 2. 0.975 gAg. Imolag 3. 87.2129-8818445 87.212-88.84.6.
107.837g/4y = 1.632g = 1.634g
-0.009 mol Ag
8.
0.6sager. Imolag
35.4539
= 0.019 mol
87.212
88.187
9.
g
0.975 8
0.009
mol
88.844 8
1.632 B
88.846 8 g
1.6348
N/A
N/A S
g
g
10.
6.0
5. 3W.KIND
g
g
mol
0.019
Agclz
40.33% %
%
%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
