Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter13: Introduction To Symmetry In Quantum Mechanics
Section: Chapter Questions
Problem 13.36E: Determine if the following species have permanent dipole moments. a Dichloromethane, CH2Cl2 b...
Related questions
Question
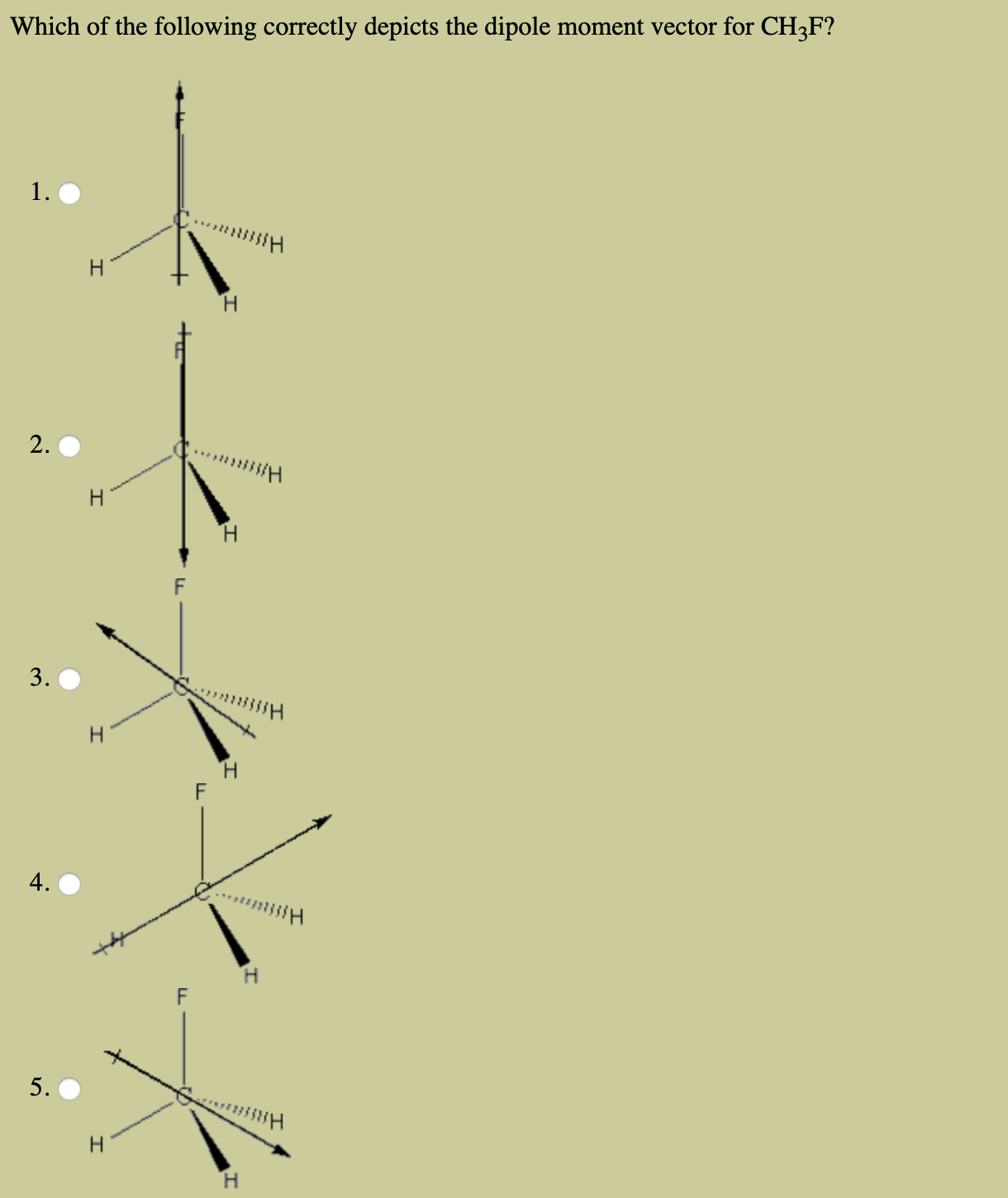
Transcribed Image Text:Which of the following correctly depicts the dipole moment vector for CH3F?
1.
H
Hissn
H
2.
H
Hiss
H
3.
H
H
F
4.
5.
H
T
T
I
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,