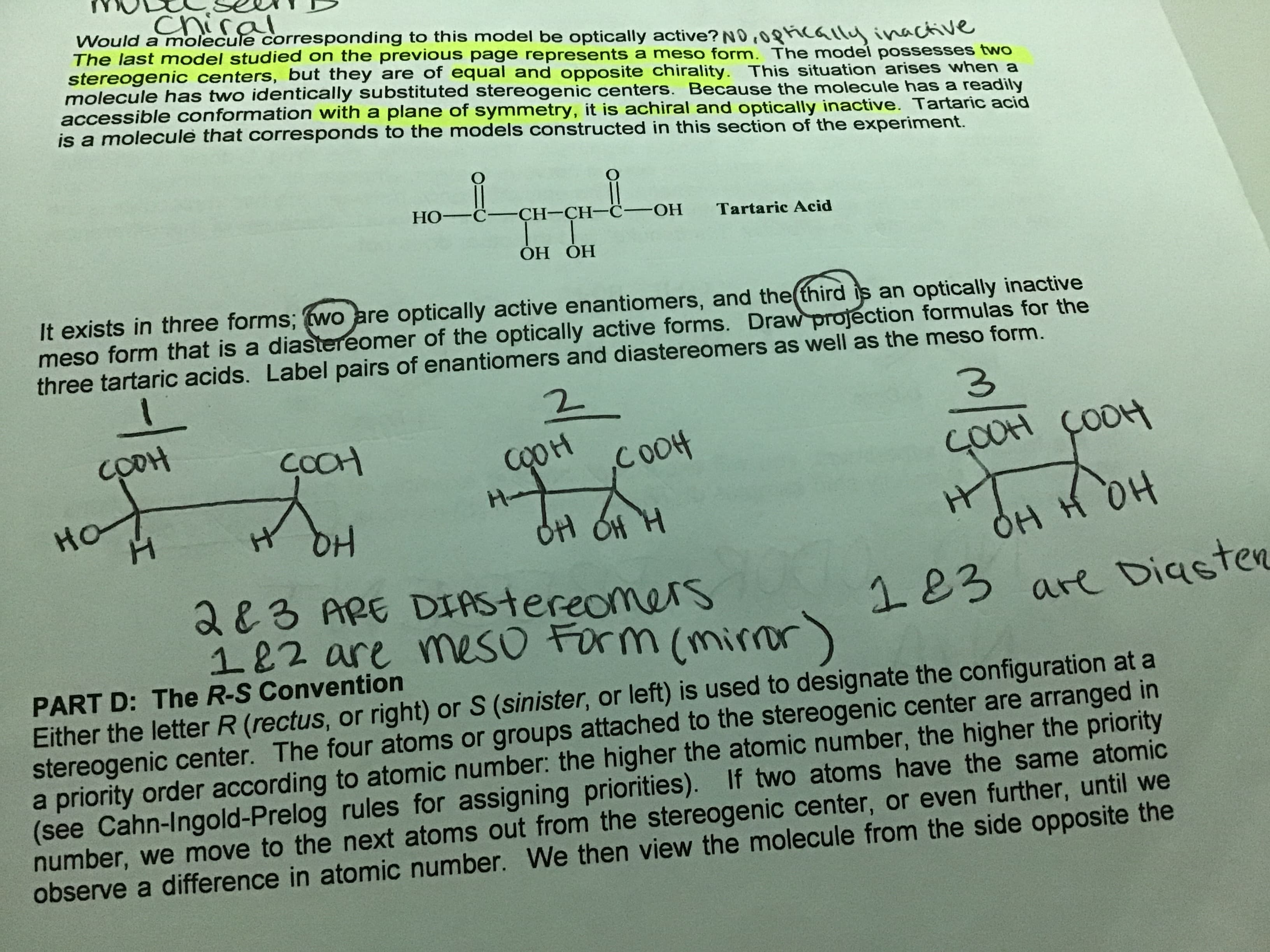
chiral Would a molecule corresponding to this model be optically active? NO,08ically inactive The last model studied on the previous page represents a meso form. The model possesses two stereogenic centers, but they are of equal and opposite chirality. This situation arises when a molecule has two identically substituted stereogenic centers. Because the molecule has a readily accessible conformation with a plane of symmetry, it is achiral and optically inactive. Tartaric acid is a molecule that corresponds to the models constructed in this section of the experiment. Но-С— СH-СH—с-ОН Tartaric Acid ОН ОН It exists in three forms; (wo are optically active enantiomers, and the(third is an optically inactive meso form that is a diastereomer of the optically active forms. Draw projection formulas for the three tartaric acids. Label pairs of enantiomers and diastereomers as well as the meso form. 3. COOH COOH COH COOH ÇOOH H- HO HT OH H OH OH OH H 283 ARE DIAStereomers 182 are mesO Form (mirror) 183 are DiasteR PART D: The R-S Convention Either the letter R (rectus, or right) or S (sinister, or left) is used to designate the configuration at a stereogenic center. The four atoms or groups attached to the stereogenic center are arranged in a priority order according to atomic number: the higher the atomic number, the higher the priority (see Cahn-Ingold-Prelog rules for assigning priorities). If two atoms have the same atomic number, we move to the next atoms out from the stereogenic center, or even further, until we observe a difference in atomic number. We then view the molecule from the side opposite the
Carbohydrates
Carbohydrates are the organic compounds that are obtained in foods and living matters in the shape of sugars, cellulose, and starch. The general formula of carbohydrates is Cn(H2O)2. The ratio of H and O present in carbohydrates is identical to water.
Starch
Starch is a polysaccharide carbohydrate that belongs to the category of polysaccharide carbohydrates.
Mutarotation
The rotation of a particular structure of the chiral compound because of the epimerization is called mutarotation. It is the repercussion of the ring chain tautomerism. In terms of glucose, this can be defined as the modification in the equilibrium of the α- and β- glucose anomers upon its dissolution in the solvent water. This process is usually seen in the chemistry of carbohydrates.
L Sugar
A chemical compound that is represented with a molecular formula C6H12O6 is called L-(-) sugar. At the carbon’s 5th position, the hydroxyl group is placed to the compound’s left and therefore the sugar is represented as L(-)-sugar. It is capable of rotating the polarized light’s plane in the direction anticlockwise. L isomers are one of the 2 isomers formed by the configurational stereochemistry of the carbohydrates.
Draw three projection formulas dor the three tartic acids.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images







