Choose a depiction of a gas sample containing equal molar amounts of argon and xenon as described by kinetic molecular theory. Red dots are used to represent argon atoms and blue dots to represent xenon atoms. Each atom is drawn with a "tail" that represents its velocity relative to the others in the mixture.
Choose a depiction of a gas sample containing equal molar amounts of argon and xenon as described by kinetic molecular theory. Red dots are used to represent argon atoms and blue dots to represent xenon atoms. Each atom is drawn with a "tail" that represents its velocity relative to the others in the mixture.
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 54A
Related questions
Question
100%
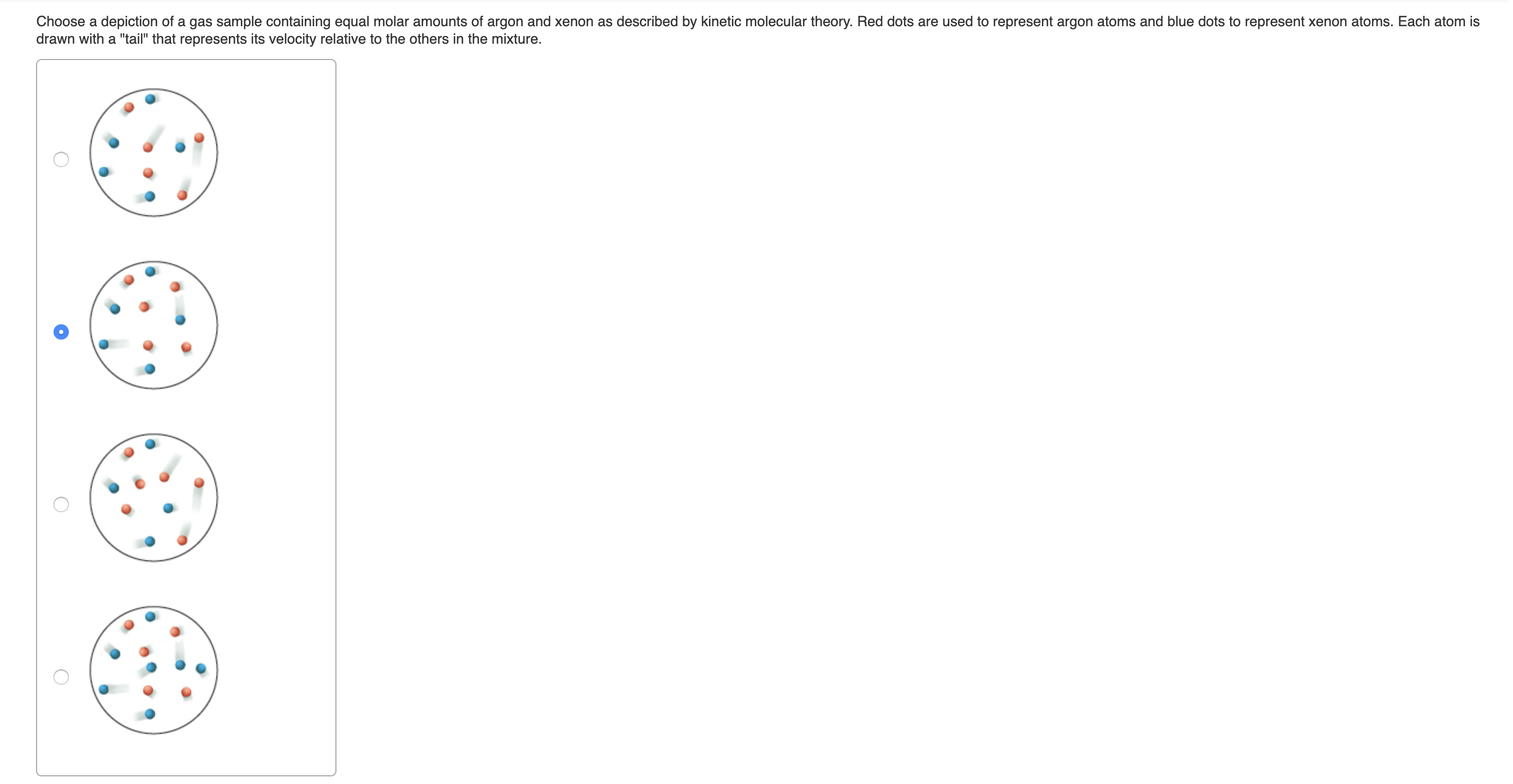
Transcribed Image Text:Choose a depiction of a gas sample containing equal molar amounts of argon and xenon as described by kinetic molecular theory. Red dots are used to represent argon atoms and blue dots to represent xenon atoms. Each atom is
drawn with a "tail" that represents its velocity relative to the others in the mixture.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Expert Answers to Latest Homework Questions
Q: Solve the following for x:
Q: Solve the following for x:
Q: Draw a Lewis structure for CH4 in which the central C atom obeys the octet rule, and answer the…
Q: How can Ava utilize relational models theory to create a collective sense of unity during the…
Q: view pictures
Q: The mayor would have had much better luck blocking the evacuation of the city, yet still receiving…
Q: Question 16
Solve for x
N
12
K
5
10
Question 17
M
Q: At the beginning of her discussion with the mayor, Ava does not even allow the mayor to make any…
Q: Determine the structure of the unknown using the data provided, thank you.
Q: In order to make distinctions between different dimensions of a problem, such as an impending…
Q: Assume Josh tends to take conflict personally. Based on this assumption and what you know about…
Q: Discuss a time when you have had to deal with a sensitive and/or a negative message. What would you…
Q: A researcher is trying to purify a protein that needs to be in a reducing environment and wants to…
Q: Describe the four c's Consistency, Coherence, Clarity, and Conclusion. Explain why they are…
Q: Iverson Company purchased a delivery truck for $45,000 on January 1, 2021. The truck was assigned an…
Q: Please help me fill in all the information
Q: Farley Corporation purchased land adjacent to its plant to improve access for trucks making…
Q: Indicate whether each of the following expenditures should be classified as land, land improvements,…
Q: Describe the top ten communication skills that business leaders search for? Explain.
Q: Determine the structure of the unknown using the data provided.
Q: Resource Allocation Podunk Institute of Technology's Math Department offers two courses: Finite…