Chp7 Rydberg1: CH NST Definitions of the S X X + Energy 4 T:TOBUR Review Determine the amount of energy in 1.00 mole of photons with a frequency of 241 GHz. (n=6.023*10^23) 1.60*10^-22 J 8.27*10A-37 J 96.2J 2.65*10^-46 J Check My Answer Show Correct Answer A Show Submitted Answer A,
Chp7 Rydberg1: CH NST Definitions of the S X X + Energy 4 T:TOBUR Review Determine the amount of energy in 1.00 mole of photons with a frequency of 241 GHz. (n=6.023*10^23) 1.60*10^-22 J 8.27*10A-37 J 96.2J 2.65*10^-46 J Check My Answer Show Correct Answer A Show Submitted Answer A,
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter7: Quantum Theory Of The Atom
Section: Chapter Questions
Problem 7.47QP: A particular transition of the rubidium atom emits light whose frequency is 3.84 1014 Hz. (Hz is...
Related questions
Question
How do I solve this? I got the correct answer before but don't remember what I did to get it
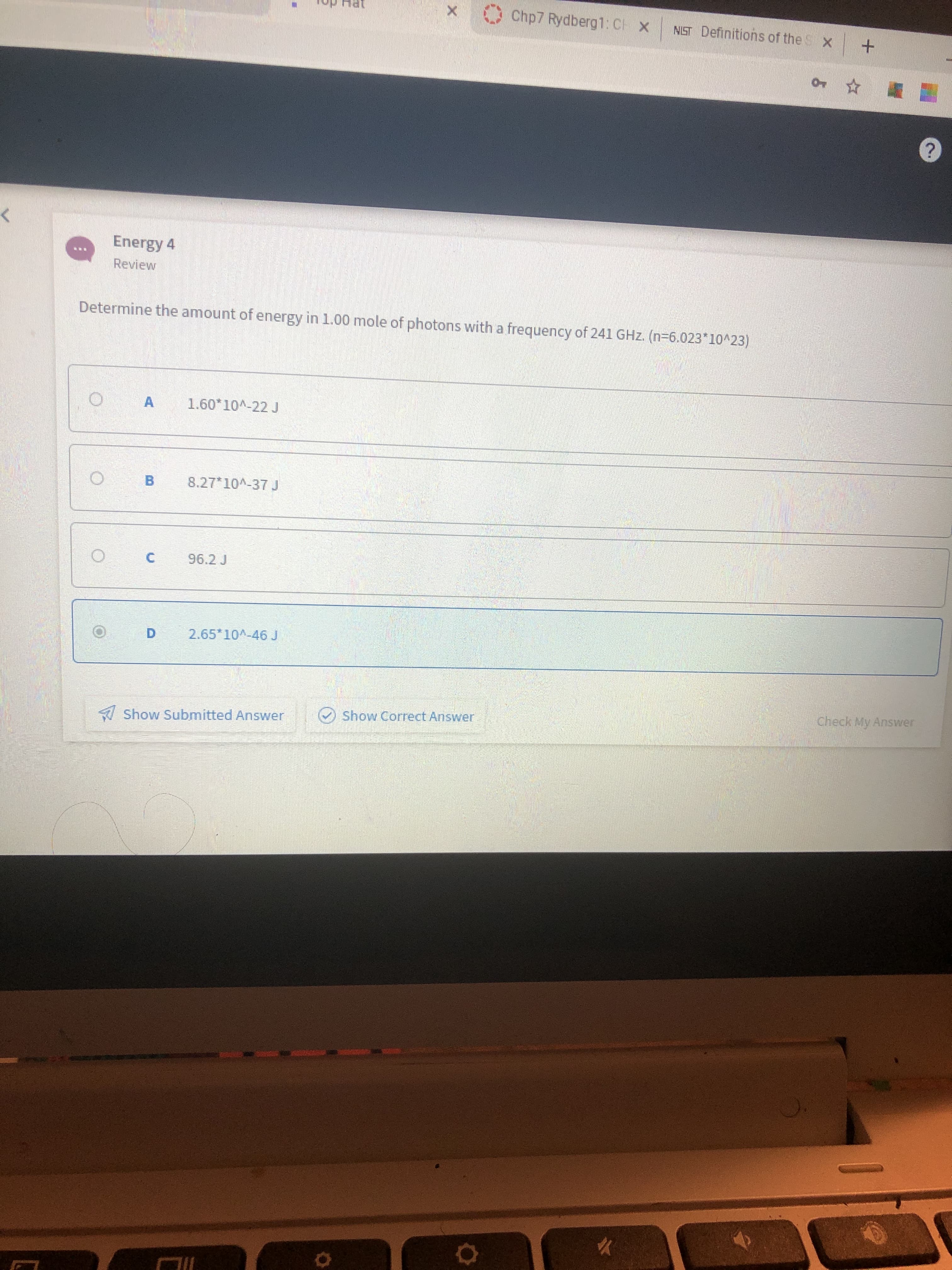
Transcribed Image Text:Chp7 Rydberg1: CH
NST Definitions of the S X
X
+
Energy 4
T:TOBUR
Review
Determine the amount of energy in 1.00 mole of photons with a frequency of 241 GHz. (n=6.023*10^23)
1.60*10^-22 J
8.27*10A-37 J
96.2J
2.65*10^-46 J
Check My Answer
Show Correct Answer
A Show Submitted Answer
A,
Expert Solution
Step 1
The energy associated with one mole of photon is given as:

Step 2
Given,
Frequency, ν = 241 GHz
Conversion of GHz to Hz :
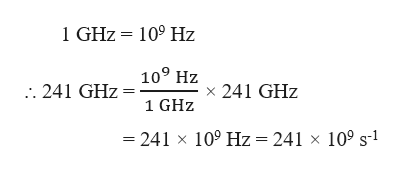
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning