Cindarium: Identity PotaSSIUm Group #_M E.C. E.C of Ion Explanation: Dosum: IdentityCalcium Group #_ E.C. E.C of Ion Explanation: Group # 2 E.C of lon Хcene: Identity Banum E.C Explanation: olu ria Lokium: Identity Soclium Group # ina E.C. E.C of Ion Explanation: nit net Jackdan: Identity Lthium Group# E.C. E.C of Ion ri Explanation: in: Eutopium: IdentityChlonide E.C Explanation: Group # E.C of Ion he ri Nastalgium: Identity Oxuaen Group #_ E.C of Ion n E.C. Explanation: i Group # 5 E.C of lon Experium: Identity trecn E.C. Explanation: 56 Identification of Alien Elements For each of the alien elements, provide the identity and group number on the periodic table. Provide an explanation of how you arrived at your conclusions. Once you have identified the element, provide an electron configuration for the element and its major ion. Attentium SymBol Identity ronium Group # E.C. E.C of lon Explanation: Mallad Group# E.C of Ion IdentityCopper E.C Explanation: 55
Cindarium: Identity PotaSSIUm Group #_M E.C. E.C of Ion Explanation: Dosum: IdentityCalcium Group #_ E.C. E.C of Ion Explanation: Group # 2 E.C of lon Хcene: Identity Banum E.C Explanation: olu ria Lokium: Identity Soclium Group # ina E.C. E.C of Ion Explanation: nit net Jackdan: Identity Lthium Group# E.C. E.C of Ion ri Explanation: in: Eutopium: IdentityChlonide E.C Explanation: Group # E.C of Ion he ri Nastalgium: Identity Oxuaen Group #_ E.C of Ion n E.C. Explanation: i Group # 5 E.C of lon Experium: Identity trecn E.C. Explanation: 56 Identification of Alien Elements For each of the alien elements, provide the identity and group number on the periodic table. Provide an explanation of how you arrived at your conclusions. Once you have identified the element, provide an electron configuration for the element and its major ion. Attentium SymBol Identity ronium Group # E.C. E.C of lon Explanation: Mallad Group# E.C of Ion IdentityCopper E.C Explanation: 55
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter7: Ionic Compounds And Metals
Section7.3: Names And Formulas For Ionic Compounds
Problem 39SSC
Related questions
Question
Cindarium

Transcribed Image Text:Cindarium: Identity PotaSSIUm Group #_M
E.C.
E.C of Ion
Explanation:
Dosum:
IdentityCalcium Group #_
E.C.
E.C of Ion
Explanation:
Group # 2
E.C of lon
Хcene:
Identity Banum
E.C
Explanation:
olu
ria
Lokium:
Identity Soclium Group #
ina
E.C.
E.C of Ion
Explanation:
nit
net
Jackdan: Identity Lthium
Group#
E.C.
E.C of Ion
ri
Explanation:
in:
Eutopium: IdentityChlonide
E.C
Explanation:
Group #
E.C of Ion
he
ri
Nastalgium: Identity Oxuaen Group #_
E.C of Ion
n
E.C.
Explanation:
i
Group # 5
E.C of lon
Experium: Identity trecn
E.C.
Explanation:
56
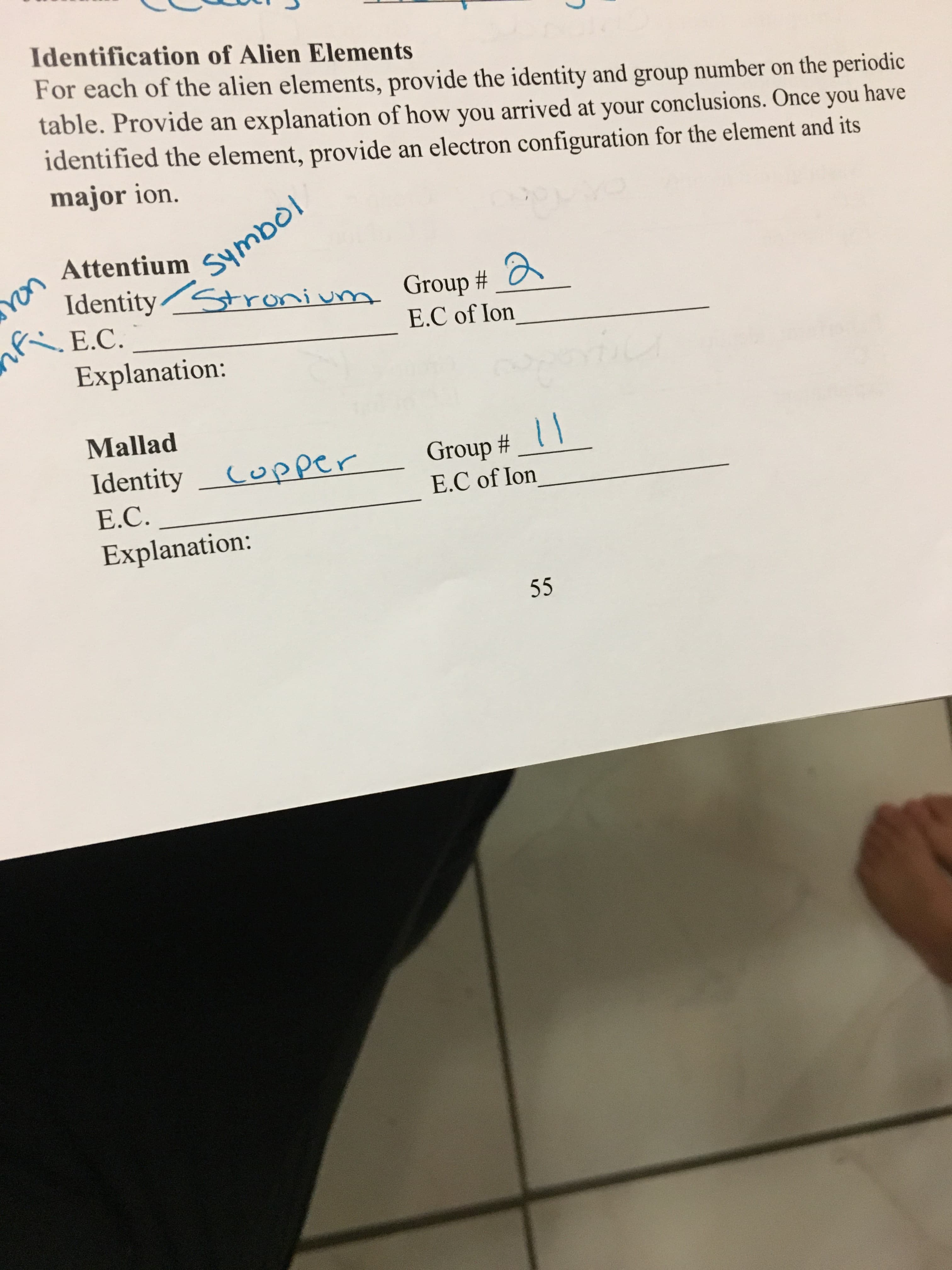
Transcribed Image Text:Identification of Alien Elements
For each of the alien elements, provide the identity and group number on the periodic
table. Provide an explanation of how you arrived at your conclusions. Once you have
identified the element, provide an electron configuration for the element and its
major ion.
Attentium SymBol
Identity ronium Group #
E.C.
E.C of lon
Explanation:
Mallad
Group#
E.C of Ion
IdentityCopper
E.C
Explanation:
55
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning