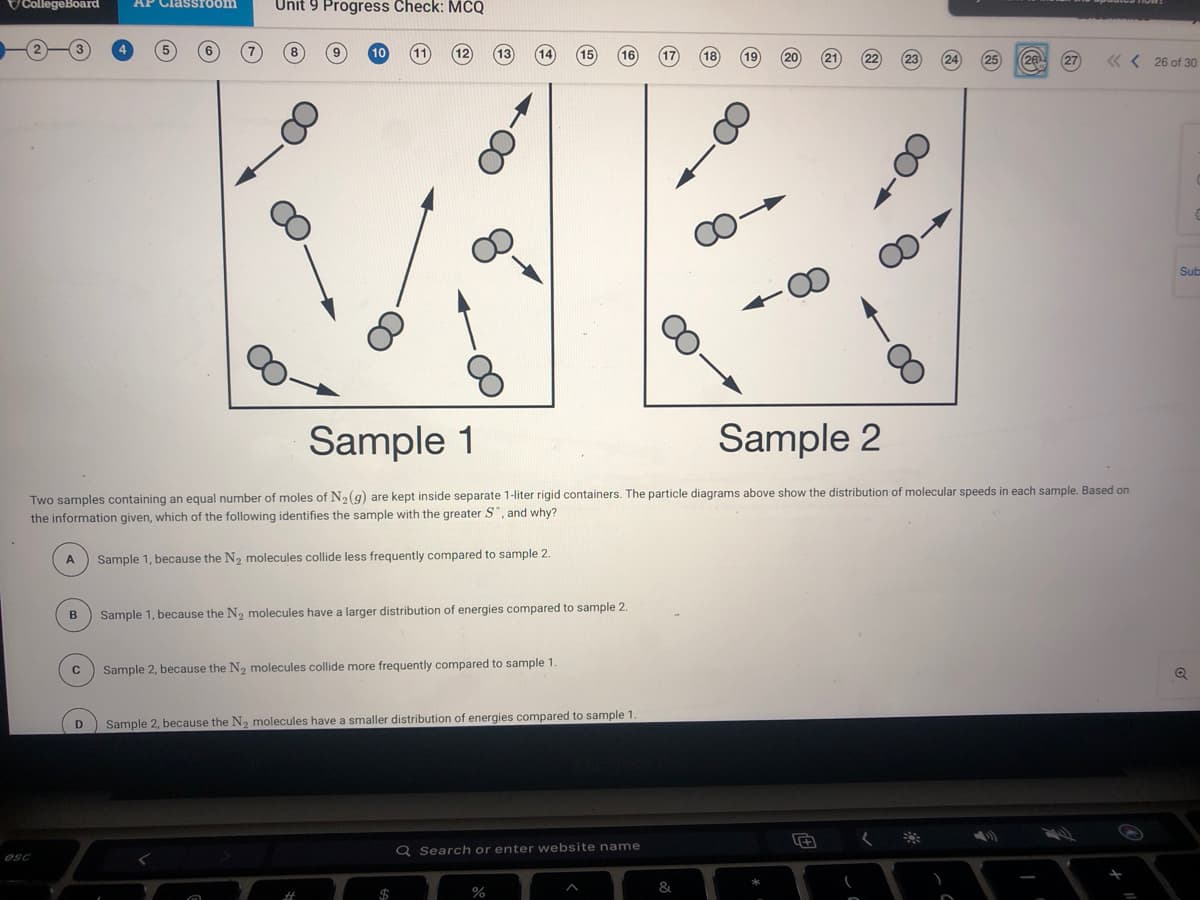
CollegeBoard AP Classroom Unit 9 Progress Check: MCQ 12 13 18 (19 (20 21 26 27 « < 26 of 30 Sub Sample 1 Sample 2 Two samples containing an equal number of moles of N(g) are kept inside separate 1-liter rigid containers. The particle diagrams above show the distribution of molecular speeds in each sample. Based on the information given, which of the following identifies the sample with the greater S", and why? Sample 1, because the N2 molecules collide less frequently compared to sample 2. B Sample 1, because the N2 molecules have a larger distribution of energies compared to sample 2. Sample 2, because the N, molecules collide more frequently compared to sample 1. Sample 2, because the N2 molecules have a smaller distribution of energies compared to sample 1.
CollegeBoard AP Classroom Unit 9 Progress Check: MCQ 12 13 18 (19 (20 21 26 27 « < 26 of 30 Sub Sample 1 Sample 2 Two samples containing an equal number of moles of N(g) are kept inside separate 1-liter rigid containers. The particle diagrams above show the distribution of molecular speeds in each sample. Based on the information given, which of the following identifies the sample with the greater S", and why? Sample 1, because the N2 molecules collide less frequently compared to sample 2. B Sample 1, because the N2 molecules have a larger distribution of energies compared to sample 2. Sample 2, because the N, molecules collide more frequently compared to sample 1. Sample 2, because the N2 molecules have a smaller distribution of energies compared to sample 1.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.118P: 5-118 Isooctane, which has a chemical formula C8H18 is the component of gasoline from which the term...
Related questions
Question
Transcribed Image Text:CollegeBoard
AP Classroom
Unit 9 Progress Check: MCQ
12
13
18
19
(20
21
26
27
« < 26 of 30
Sub
Sample 1
Sample 2
Two samples containing an equal number of moles of N(g) are kept inside separate 1-liter rigid containers. The particle diagrams above show the distribution of molecular speeds in each sample. Based on
the information given, which of the following identifies the sample with the greater S", and why?
Sample 1, because the N2 molecules collide less frequently compared to sample 2.
B
Sample 1, because the N2 molecules have a larger distribution of energies compared to sample 2.
Sample 2, because the N, molecules collide more frequently compared to sample 1.
Sample 2, because the N2 molecules have a smaller distribution of energies compared to sample 1
Q Search or enter website name
esc
$
%
&
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
