Complete the following for these redox reactions: a. determine the oxidation number of each element; list the appropriate oxidation number above every element in the entire chemical equation b. identify which species (atom or ion) that is being oxidized in the reaction (oxidation number is increasing from reactant side to product side) c. which species (atom or ion) that is being reduced in the reaction (oxidation number is decreasing from reactant side to product side) d. which reactant is considered the oxidizing agent (contains the species being reduced) e. which reactant is considered the reducing agent (contains the species being oxidized) i. Mn 2+ ii. Clo + Cr(OH)4¯ iii. NO3 + SO2 + ClO3→ MnO2 + ClO2 → CrO4 2- SO,2- + NO2 iv. Zn + NO3- → NH3 + Zn(OH)4² + Cl 2-
Complete the following for these redox reactions: a. determine the oxidation number of each element; list the appropriate oxidation number above every element in the entire chemical equation b. identify which species (atom or ion) that is being oxidized in the reaction (oxidation number is increasing from reactant side to product side) c. which species (atom or ion) that is being reduced in the reaction (oxidation number is decreasing from reactant side to product side) d. which reactant is considered the oxidizing agent (contains the species being reduced) e. which reactant is considered the reducing agent (contains the species being oxidized) i. Mn 2+ ii. Clo + Cr(OH)4¯ iii. NO3 + SO2 + ClO3→ MnO2 + ClO2 → CrO4 2- SO,2- + NO2 iv. Zn + NO3- → NH3 + Zn(OH)4² + Cl 2-
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 10RQ
Related questions
Question
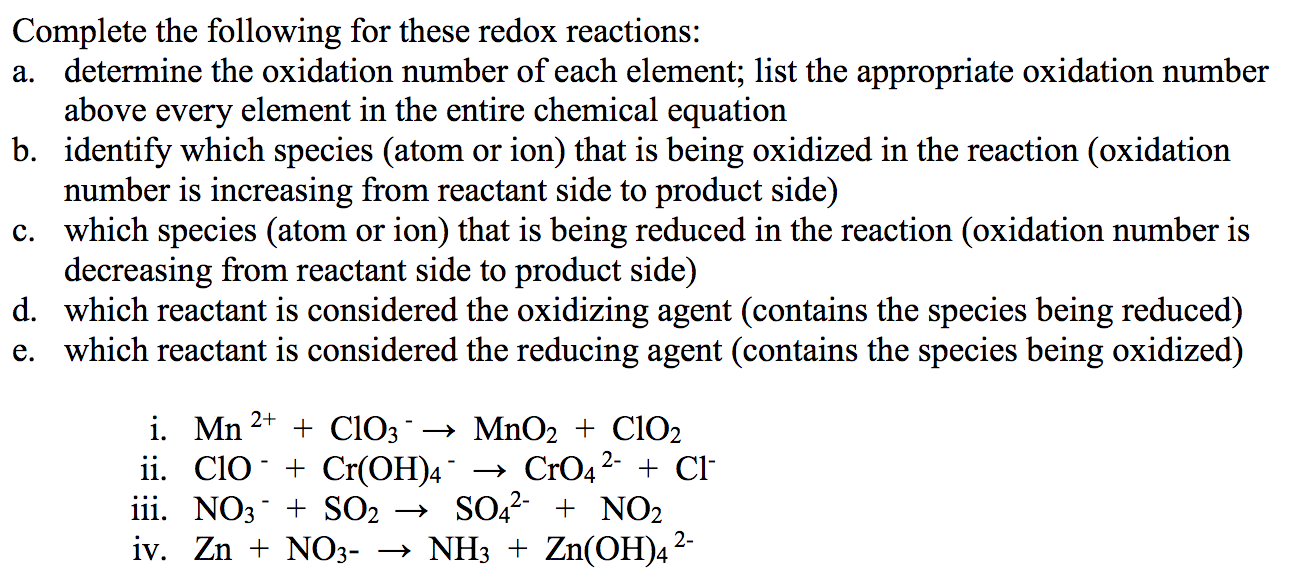
Transcribed Image Text:Complete the following for these redox reactions:
a. determine the oxidation number of each element; list the appropriate oxidation number
above every element in the entire chemical equation
b. identify which species (atom or ion) that is being oxidized in the reaction (oxidation
number is increasing from reactant side to product side)
c. which species (atom or ion) that is being reduced in the reaction (oxidation number is
decreasing from reactant side to product side)
d. which reactant is considered the oxidizing agent (contains the species being reduced)
e. which reactant is considered the reducing agent (contains the species being oxidized)
i. Mn 2+
ii. Clo + Cr(OH)4¯
iii. NO3 + SO2
+ ClO3→ MnO2 + ClO2
→ CrO4 2-
SO,2- + NO2
iv. Zn + NO3- → NH3 + Zn(OH)4²
+ Cl
2-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 6 images

Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning