Complete the sentences to explain your answers. Reset Help dense According to Rutherford's nuclear theory, most of the volume of an atom is so the volume of a hydrogen atom cannot be mostly due to the proton. less than negatively charged According to Rutherford's nuclear theory, the core of an atom (nucleus) contains most of the of an atom and is so the majority of the mass of a fluorine atom positively charged cannot be due to its nine electrons. the same as According to Rutherford's nuclear theory, the number of negatively charged particles outside the more than nucleus is the number of positively charged particles within the nucleus, so a nitrogen particles atom has 7 protons and 7 electrons, while a phosphorous atom cannot have 15 protons and 150 empty space electrons mass
Complete the sentences to explain your answers. Reset Help dense According to Rutherford's nuclear theory, most of the volume of an atom is so the volume of a hydrogen atom cannot be mostly due to the proton. less than negatively charged According to Rutherford's nuclear theory, the core of an atom (nucleus) contains most of the of an atom and is so the majority of the mass of a fluorine atom positively charged cannot be due to its nine electrons. the same as According to Rutherford's nuclear theory, the number of negatively charged particles outside the more than nucleus is the number of positively charged particles within the nucleus, so a nitrogen particles atom has 7 protons and 7 electrons, while a phosphorous atom cannot have 15 protons and 150 empty space electrons mass
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section: Chapter Questions
Problem 106AP: lement X, which has a valence shell configuration of ns2np4 , was isolated in a laboratory. Which of...
Related questions
Question
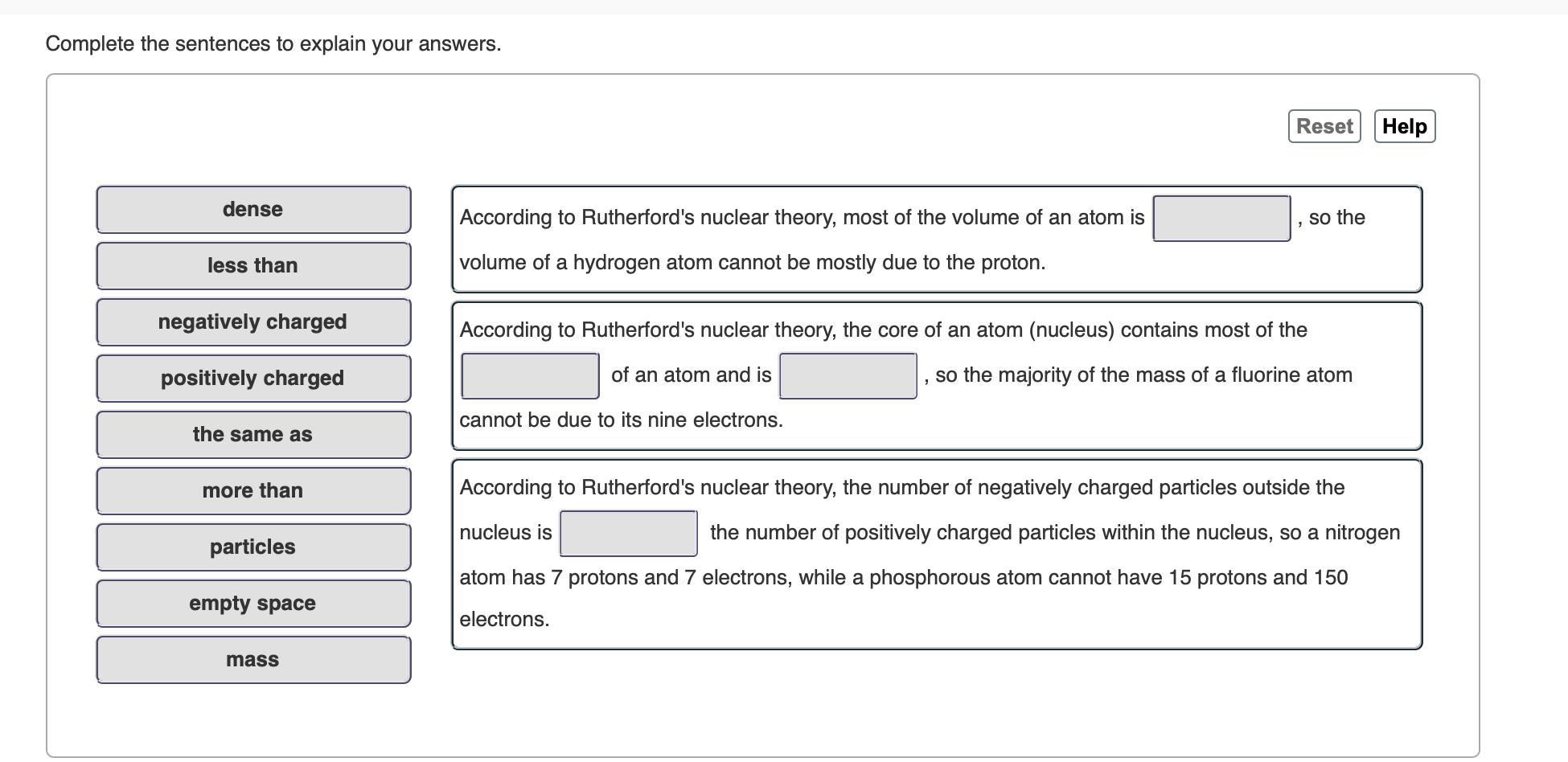
Transcribed Image Text:Complete the sentences to explain your answers.
Reset Help
dense
According to Rutherford's nuclear theory, most of the volume of an atom is
so the
volume of a hydrogen atom cannot be mostly due to the proton.
less than
negatively charged
According to Rutherford's nuclear theory, the core of an atom (nucleus) contains most of the
of an atom and is
so the majority of the mass of a fluorine atom
positively charged
cannot be due to its nine electrons.
the same as
According to Rutherford's nuclear theory, the number of negatively charged particles outside the
more than
nucleus is
the number of positively charged particles within the nucleus, so a nitrogen
particles
atom has 7 protons and 7 electrons, while a phosphorous atom cannot have 15 protons and 150
empty space
electrons
mass
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning