Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. Does a empirical formula of precipitate form when A and B solution A solution B precipitate are mixed? X 6 Oyes no zinc nitrate iron(II) chloride yes silver nitrate sodium bromide no yes barium nitrate sodium chloride Check Explanation Privacy Terms of Use 2019 McGraw-Hill Education. All Rights Reserved. MacBook Pro DII F10 F9 O00 O00 F8 F7 F6 F5 F 4 F3 esc F2 F1 8.n no
Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. Does a empirical formula of precipitate form when A and B solution A solution B precipitate are mixed? X 6 Oyes no zinc nitrate iron(II) chloride yes silver nitrate sodium bromide no yes barium nitrate sodium chloride Check Explanation Privacy Terms of Use 2019 McGraw-Hill Education. All Rights Reserved. MacBook Pro DII F10 F9 O00 O00 F8 F7 F6 F5 F 4 F3 esc F2 F1 8.n no
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 7QAP: Decide whether a precipitate will form when the following solutions are mixed. If a precipitate...
Related questions
Question
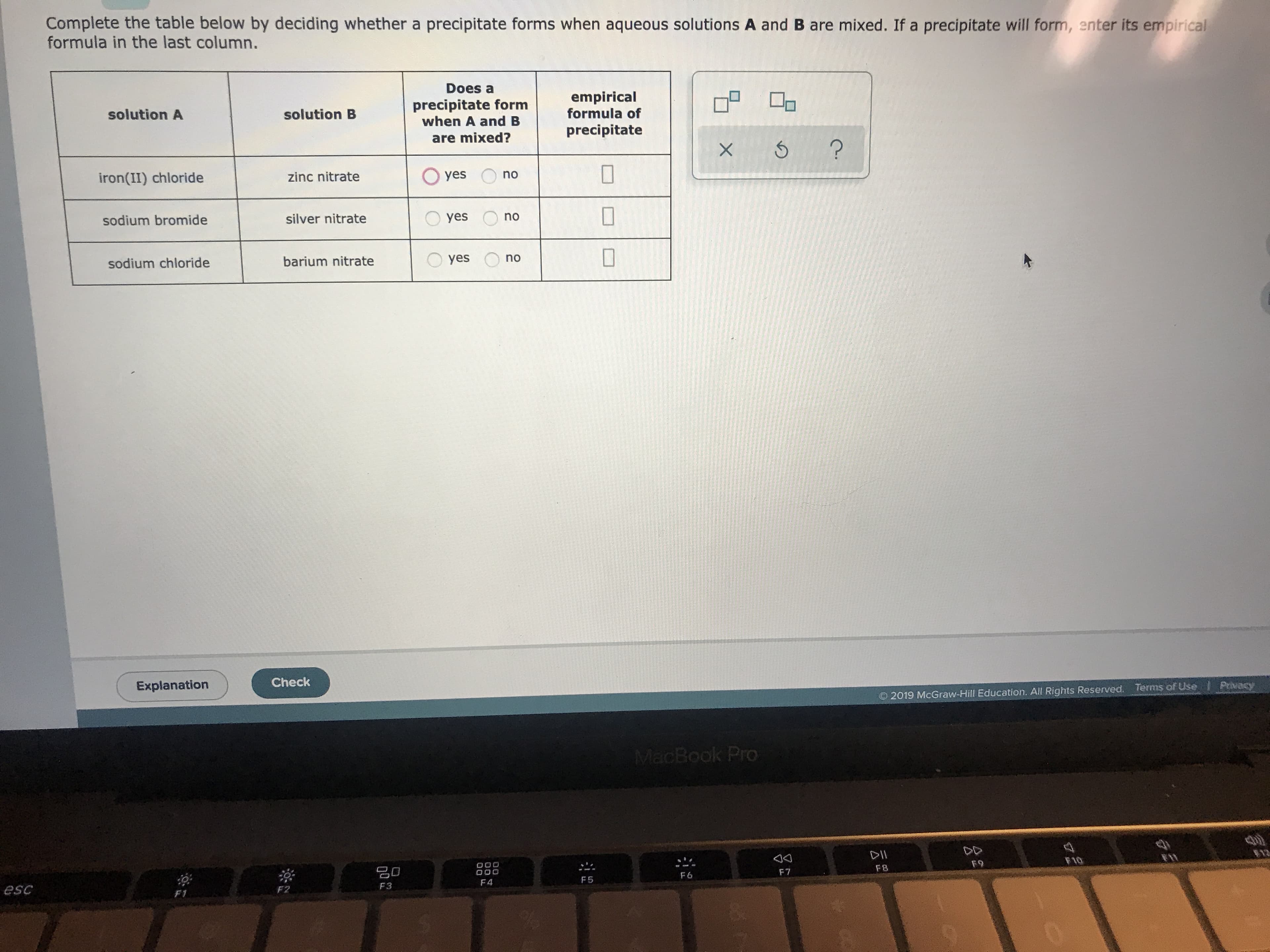
Transcribed Image Text:Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical
formula in the last column.
Does a
empirical
formula of
precipitate form
when A and B
solution A
solution B
precipitate
are mixed?
X 6
Oyes
no
zinc nitrate
iron(II) chloride
yes
silver nitrate
sodium bromide
no
yes
barium nitrate
sodium chloride
Check
Explanation
Privacy
Terms of Use
2019 McGraw-Hill Education. All Rights Reserved.
MacBook Pro
DII
F10
F9
O00
O00
F8
F7
F6
F5
F 4
F3
esc
F2
F1
8.n
no
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning