Consider an introductory thermodynamics class experiment used to demonstrate phase change phenomena. A beaker of water is heated, and its temperature measured over time to establish the temperature at which boiling occurs. The results, shown in the Figure below, are for three separate tests conducted on different days by different student groups using the same equipment and method. Why might the data from three seemingly identical tests show different results? Temperature (°C) 101 100- 99- 98- 97- 96 5 95 94 1 No 2 4 Time (min) Boiling region 5 100.3 100.1 99.8 Boiling point results Test 1 (762 mm Hg) Test 2 (754 mm Hg) -Test 3 (767 mm Hg) 6
Consider an introductory thermodynamics class experiment used to demonstrate phase change phenomena. A beaker of water is heated, and its temperature measured over time to establish the temperature at which boiling occurs. The results, shown in the Figure below, are for three separate tests conducted on different days by different student groups using the same equipment and method. Why might the data from three seemingly identical tests show different results? Temperature (°C) 101 100- 99- 98- 97- 96 5 95 94 1 No 2 4 Time (min) Boiling region 5 100.3 100.1 99.8 Boiling point results Test 1 (762 mm Hg) Test 2 (754 mm Hg) -Test 3 (767 mm Hg) 6
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
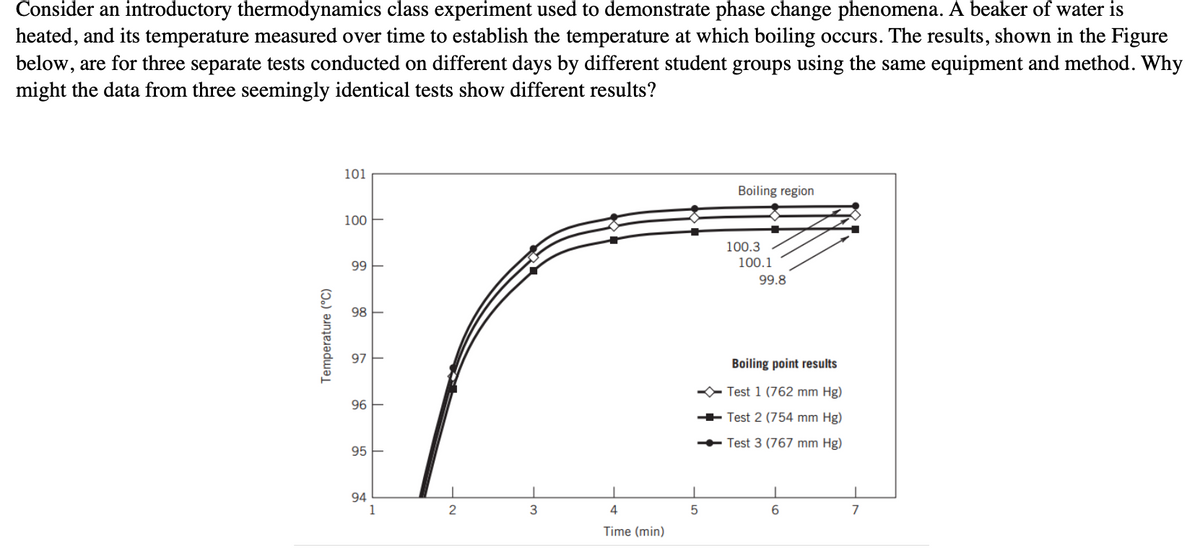
Transcribed Image Text:Consider an introductory thermodynamics class experiment used to demonstrate phase change phenomena. A beaker of water is
heated, and its temperature measured over time to establish the temperature at which boiling occurs. The results, shown in the Figure
below, are for three separate tests conducted on different days by different student groups using the same equipment and method. Why
might the data from three seemingly identical tests show different results?
Temperature (°C)
101
100
99
98
97
96
95
94
1
2
3
4
Time (min)
5
Boiling region
100.3
100.1
99.8
Boiling point results
Test 1 (762 mm Hg)
Test 2 (754 mm Hg)
Test 3 (767 mm Hg)
6
7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY