Consider the reaction 2 NO (g) + H2 (g) ⇌ N2O (g) + H2O (g) with ΔH° = + 36 kJ. In which direction, left or right, will the equilibrium shift if the following changes are made? 1. NO is added ________________________ 2. the system is cooled ________________________ 3. H2 is removed ________________________
Consider the reaction 2 NO (g) + H2 (g) ⇌ N2O (g) + H2O (g) with ΔH° = + 36 kJ. In which direction, left or right, will the equilibrium shift if the following changes are made? 1. NO is added ________________________ 2. the system is cooled ________________________ 3. H2 is removed ________________________
Chapter8: Reaction Rates And Equilibrium
Section: Chapter Questions
Problem 8.93E
Related questions
Question
Consider the reaction 2 NO (g) + H2 (g) ⇌ N2O (g) + H2O (g) with ΔH° = + 36 kJ. In
which direction, left or right, will the equilibrium shift if the following changes are
made?
1. NO is added ________________________
2. the system is cooled ________________________
3. H2 is removed ________________________
4. pressure is increased ________________________
5. N2O is added ________________________
6. H2 is removed _______________________
explain your answer.
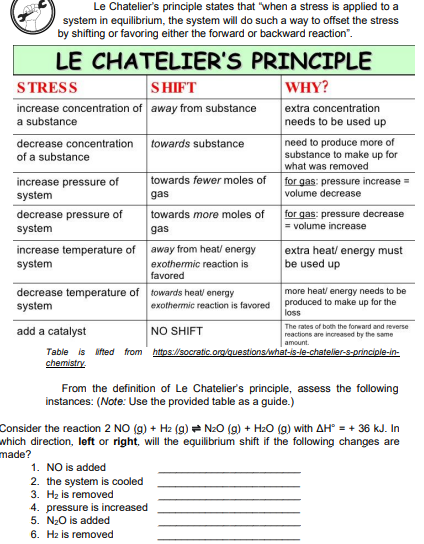
Transcribed Image Text:Le Chatelier's principle states that "when a stress is applied to a
system in equilibrium, the system will do such a way to offset the stress
by shifting or favoring either the forward or backward reaction".
LE CHATELIER'S PRINCIPLE
S HIFT
increase concentration of away from substance
STRESS
WHY?
extra concentration
needs to be used up
a substance
decrease concentration towards substance
of a substance
need to produce more of
substance to make up for
what was removed
increase pressure of
system
towards fewer moles of
for gas: pressure increase =
volume decrease
gas
decrease pressure of
towards more moles of
for gas: pressure decrease
= volume increase
system
gas
increase temperature of away from heat/ energy
extra heat/ energy must
be used up
system
exothermic reaction is
favored
decrease temperature of towards heat/ energy
system
more heat/ energy needs to be
produced to make up for the
loss
exothermic reaction is favored
The rates of both the forward and reverse
add a catalyst
NO SHIFT
reactions are increased by the same
amount.
Table is lifted from httos://socratic.org/questions/what-is-le-chateller-s-principle-in-
chemistry.
From the definition of Le Chatelier's principle, assess the following
instances: (Note: Use the provided table as a guide.)
Consider the reaction 2 NO (g) + Hz (g) = N2O (g) + H2O (g) with AH° = + 36 kJ. In
which direction, left or right, will the equilibrium shift if the following changes are
made?
1. NO is added
2. the system is cooled
3. Hz is removed
4. pressure is increased
5. N20 is added
6. Hz is removed
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning