Consider the reaction C12 H22O1 (s) +1202 (g)+12C02 (g)+ 11H20(1) in which 10.0 g of sucrose, C12 H22O11, was burned in a bomb calorimeter with a heat capacity of 7.50 kJ/ C. The temperature increase inside the calorimeter was found to be 22.0 °C. Calculate the change in internal energy, AE, for this reaction per mole of sucrose. Express the change in internal energy in kilojoules per mole to three significant figures. • View Available Hint(s) kJ/mol AE = Submit Previous Answers
Consider the reaction C12 H22O1 (s) +1202 (g)+12C02 (g)+ 11H20(1) in which 10.0 g of sucrose, C12 H22O11, was burned in a bomb calorimeter with a heat capacity of 7.50 kJ/ C. The temperature increase inside the calorimeter was found to be 22.0 °C. Calculate the change in internal energy, AE, for this reaction per mole of sucrose. Express the change in internal energy in kilojoules per mole to three significant figures. • View Available Hint(s) kJ/mol AE = Submit Previous Answers
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.79PAE: A student performing a calorimetry experiment combined 100.0 ml. of 0.50 M HCI and 100.0 ml. of 0.50...
Related questions
Question
Transcribed Image Text:Chapter 6 Ubjectives
+ Calorimetry
27 of 42
Constants Periodic Table
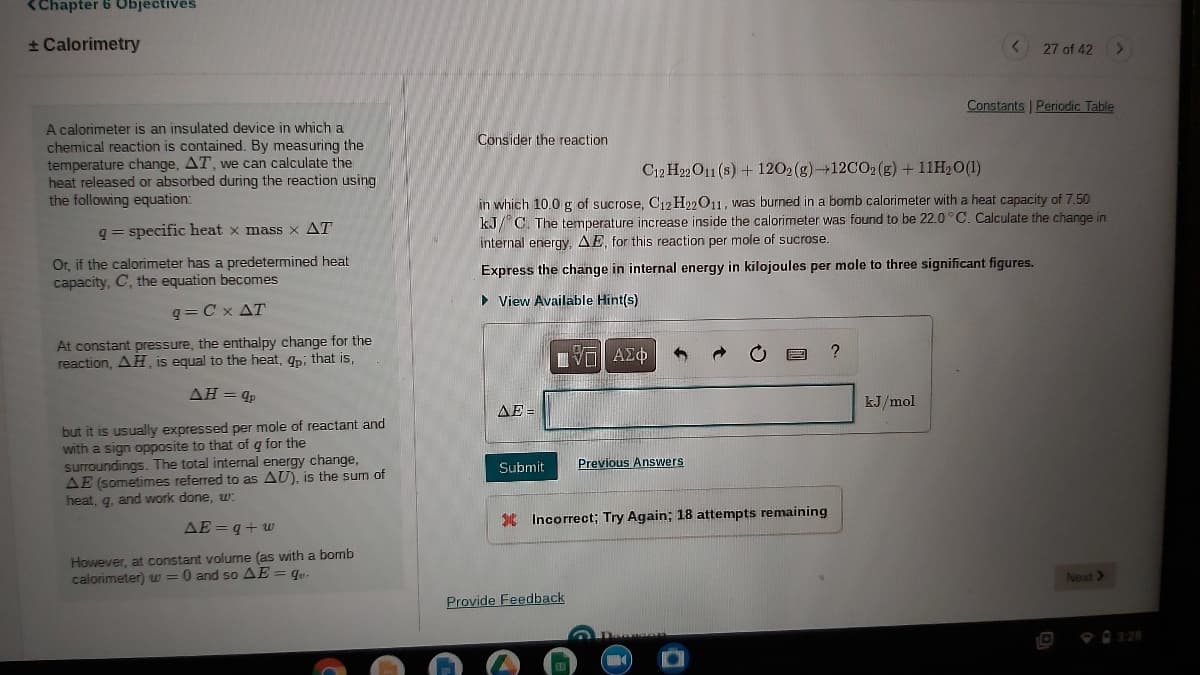
A calorimeter is an insulated device in which a
chemical reaction is contained. By measuring the
temperature change, AT, we can calculate the
heat released or absorbed during the reaction using
the following equation:
Consider the reaction
C12 H22 O11 (s) + 1202 (g)+12C02 (g) + 11H20(1)
in which 10.0 g of sucrose, C12 H22011, was burned in a bomb calorimeter with a heat capacity of 7.50
kJ/ C. The temperature increase inside the calorimeter was found to be 22.0°C. Calculate the change in
internal energy. AE, for this reaction per mole of sucrose.
q= specific heat x mass x AT
Or, if the calorimeter has a predetermined heat
capacity, C, the equation becomes
Express the change in internal energy in kilojoules per mole to three significant figures.
• View Available Hint(s)
q = C x AT
At constant pressure, the enthalpy change for the
reaction, AH is equal to the heat, qpi that is,
AH= qp
kJ/mol
AE =
but it is usually expressed per mole of reactant and
with a sign opposite to that of q for the
surroundings. The total internal energy change,
AE (sometimes referred to as AU), is the sum of
heat, g, and work done, w:
Submit
Previous Answers
* Incorrect; Try Again; 18 attempts remaining
AE = q+ w
However, at constant volume (as with a bomb
calorimeter) w =0 and so AE = qu.
Next>
Provide Feedback
3.28
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
