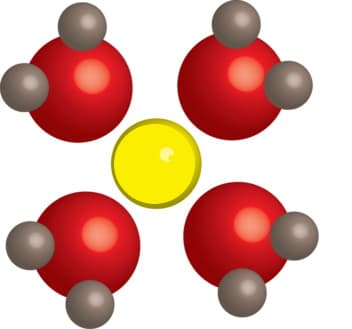
Consider this picture depicting the hydration of an atom or ion by water molecules. Of the choices below, what is the only atom or ion that the yellow particle in the center can be? Group of answer choices A:K+ ion B:oxygen atom in ethanol (CH3CH2OH) C:Cl- ion D:nitrogen atom in ammonia (NH3) The solubility of sulfur hexafluoride (SF6) gas in water is 2.1 x 10-4 M at 20 oC. Based on the choices below and without doing any calculations, what is the most likely solubility of SF6 if the solution temperature is lowered to 5 oC? Group of answer choices A:2.1 x 10-4 M B:5.6 x 10-4 M C:2.1 x 10-6 M D:1.0 x 10-4 M
Consider this picture depicting the hydration of an atom or ion by water molecules. Of the choices below, what is the only atom or ion that the yellow particle in the center can be? Group of answer choices A:K+ ion B:oxygen atom in ethanol (CH3CH2OH) C:Cl- ion D:nitrogen atom in ammonia (NH3) The solubility of sulfur hexafluoride (SF6) gas in water is 2.1 x 10-4 M at 20 oC. Based on the choices below and without doing any calculations, what is the most likely solubility of SF6 if the solution temperature is lowered to 5 oC? Group of answer choices A:2.1 x 10-4 M B:5.6 x 10-4 M C:2.1 x 10-6 M D:1.0 x 10-4 M
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter8: Solutions
Section: Chapter Questions
Problem 8.6EP: For each of the following pairs of solutions, select the solution for which solute solubility is...
Related questions
Question
Consider this picture depicting the hydration of an atom or ion by water molecules. Of the choices below, what is the only atom or ion that the yellow particle in the center can be?
Group of answer choices
A:K+ ion
B:oxygen atom in ethanol (CH3CH2OH)
C:Cl- ion
D:nitrogen atom in ammonia (NH3)
The solubility of sulfur hexafluoride (SF6) gas in water is 2.1 x 10-4 M at 20 oC. Based on the choices below and without doing any calculations, what is the most likely solubility of SF6 if the solution temperature is lowered to 5 oC?
Group of answer choices
A:2.1 x 10-4 M
B:5.6 x 10-4 M
C:2.1 x 10-6 M
D:1.0 x 10-4 M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning