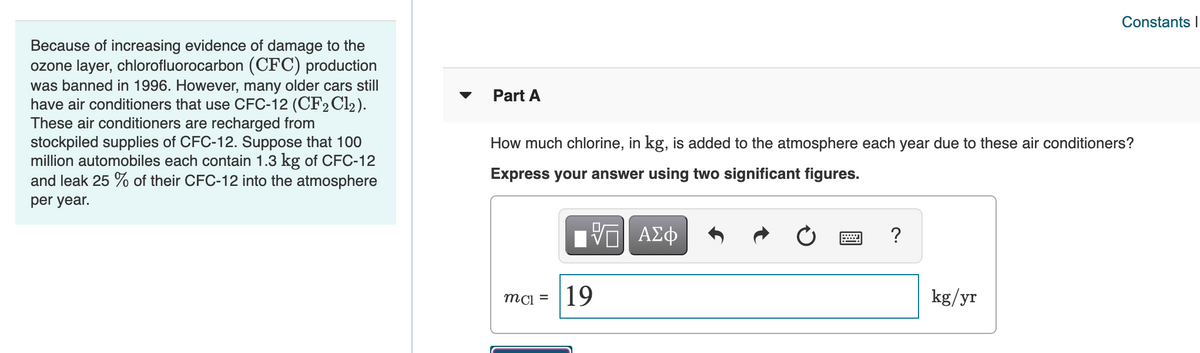
Constants Because of increasing evidence of damage to the ozone layer, chlorofluorocarbon (CFC) production was banned in 1996. However, many older cars still have air conditioners that use CFC-12 (CF2C12). These air conditioners are recharged from stockpiled supplies of CFC-12. Suppose that 100 million automobiles each contain 1.3 kg of CFC-12 and leak 25 % of their CFC-12 into the atmosphere Part A How much chlorine, in kg, is added to the atmosphere each year due to these air conditioners? Express your answer using two significant figures. per year. 1 ΑΣφ ? mci = |19 kg/yr
Constants Because of increasing evidence of damage to the ozone layer, chlorofluorocarbon (CFC) production was banned in 1996. However, many older cars still have air conditioners that use CFC-12 (CF2C12). These air conditioners are recharged from stockpiled supplies of CFC-12. Suppose that 100 million automobiles each contain 1.3 kg of CFC-12 and leak 25 % of their CFC-12 into the atmosphere Part A How much chlorine, in kg, is added to the atmosphere each year due to these air conditioners? Express your answer using two significant figures. per year. 1 ΑΣφ ? mci = |19 kg/yr
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter5: Gases
Section: Chapter Questions
Problem 130E
Related questions
Question
Transcribed Image Text:Constants I
Because of increasing evidence of damage to the
ozone layer, chlorofluorocarbon (CFC) production
was banned in 1996. However, many older cars still
have air conditioners that use CFC-12 (CF2 Cl2).
These air conditioners are recharged from
stockpiled supplies of CFC-12. Suppose that 100
million automobiles each contain 1.3 kg of CFC-12
and leak 25 % of their CFC-12 into the atmosphere
Part A
How much chlorine, in kg, is added to the atmosphere each year due to these air conditioners?
Express your answer using two significant figures.
per year.
ΑΣφ
mci =
19
kg/yr
Expert Solution
Step 1
The number of cars that using CFC-12 is = 100 million
The mass of CFC-12 used by each car is = 1.3 kg
The leakage of CFC-12 per year is = 25 %
The molecular formula of CFC-12 is = CF2Cl2
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning