Course Contents » ... » Homework Set #4 » t10p09 Timer Notes Evaluate Feedback Print Info An industrial chemist puts 2.00 mol each of H2(g) and CO,(g) in a 1.00-L container at a certain temperature. When equilibrium is reached, 0.64 mol of CO(g) is in the container. Find Keg at this temperature for the following reaction: H2(g) + CO2(g)¬H20(g) + CO(g) Submit Answer Tries 0/99 This discussion is closed. Send Feedback
Course Contents » ... » Homework Set #4 » t10p09 Timer Notes Evaluate Feedback Print Info An industrial chemist puts 2.00 mol each of H2(g) and CO,(g) in a 1.00-L container at a certain temperature. When equilibrium is reached, 0.64 mol of CO(g) is in the container. Find Keg at this temperature for the following reaction: H2(g) + CO2(g)¬H20(g) + CO(g) Submit Answer Tries 0/99 This discussion is closed. Send Feedback
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter8: Reaction Rates And Equilibrium
Section: Chapter Questions
Problem 8.49E: Consider the following equilibrium constants. Describe how you would expect the equilibrium...
Related questions
Question
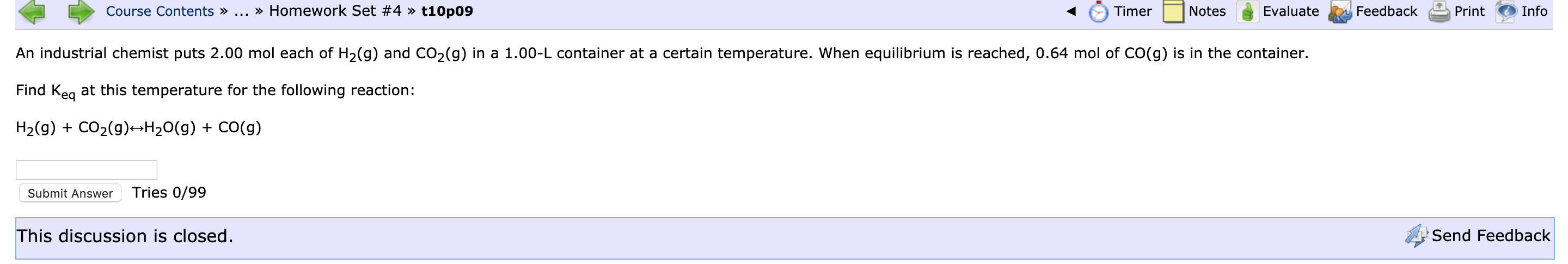
Transcribed Image Text:Course Contents » ...
» Homework Set #4 » t10p09
Timer
Notes
Evaluate
Feedback
Print
Info
An industrial chemist puts 2.00 mol each of H2(g) and CO,(g) in a 1.00-L container at a certain temperature. When equilibrium is reached, 0.64 mol of CO(g) is in the container.
Find Keg at this temperature for the following reaction:
H2(g) + CO2(g)¬H20(g) + CO(g)
Submit Answer
Tries 0/99
This discussion is closed.
Send Feedback
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 5 images

Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning