CREATE A CEPT MAP FROM THIS PASSAGE: In this article, we will discuss polyatomic ions. The prefix poly means many, so a polyatomic ion is an ion that contains more than one atom. This differentiates polyatomic ions from monatomic ions, which contain only one atom. Examples of monatomic ions include Na¹, Fe, Cl and many, many others. We can think about polyatomic ions by comparing them to monatomic ions. A monatomic ion is an atom that has been ionized by gaining or losing electrons. The ion has a net charge because the total number of electrons is not balanced by the total number of protons in the nucleus. Thus, compared to the neutral atom, we have extra electrons-in the case of a negatively charged anion-or not enough electrons-in the case of a positively charged cation. Similarly, we can think of a polyatomic ion as a molecule that has been ionized by gaining or losing electrons. In a polyatomic ion, the group of covalently bonded atoms carries a net charge because the total number of electrons in the molecule is not equal to the total number of protons in the molecule. Examples of polyatomic ions include NH, CO,2, PO,, and many others.
CREATE A CEPT MAP FROM THIS PASSAGE: In this article, we will discuss polyatomic ions. The prefix poly means many, so a polyatomic ion is an ion that contains more than one atom. This differentiates polyatomic ions from monatomic ions, which contain only one atom. Examples of monatomic ions include Na¹, Fe, Cl and many, many others. We can think about polyatomic ions by comparing them to monatomic ions. A monatomic ion is an atom that has been ionized by gaining or losing electrons. The ion has a net charge because the total number of electrons is not balanced by the total number of protons in the nucleus. Thus, compared to the neutral atom, we have extra electrons-in the case of a negatively charged anion-or not enough electrons-in the case of a positively charged cation. Similarly, we can think of a polyatomic ion as a molecule that has been ionized by gaining or losing electrons. In a polyatomic ion, the group of covalently bonded atoms carries a net charge because the total number of electrons in the molecule is not equal to the total number of protons in the molecule. Examples of polyatomic ions include NH, CO,2, PO,, and many others.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter2: Chemical Compounds
Section: Chapter Questions
Problem 118QRT
Related questions
Question
100%
Create a concept map from the passage attached. Add personalization. Example of a concept map is attached.

Transcribed Image Text:CREATE A CONCEPT MAP FROM THIS PASSAGE:
In this article, we will discuss polyatomic ions. The prefix
poly- means many, so a polyatomic ion is an ion that contains
more than one atom. This differentiates polyatomic ions from
monatomic ions, which contain only one atom. Examples of
monatomic ions include Na¹, Fe³, Cl¹ and many, many others.
We can think about polyatomic ions by comparing them to
monatomic ions. A monatomic ion is an atom that has been ionized
by gaining or losing electrons. The ion has a net charge because
the total number of electrons is not balanced by the total number
of protons in the nucleus. Thus, compared to the neutral atom, we
have extra electrons-in the case of a negatively charged anion-or
not enough electrons-in the case of a positively charged cation.
Similarly, we can think of a polyatomic ion as a molecule that has
been ionized by gaining or losing electrons. In a polyatomic ion,
the group of covalently bonded atoms carries a net charge
because the total number of electrons in the molecule is not equal
to the total number of protons in the molecule. Examples of
polyatomic ions include NH₂, CO², PO³, and many others.
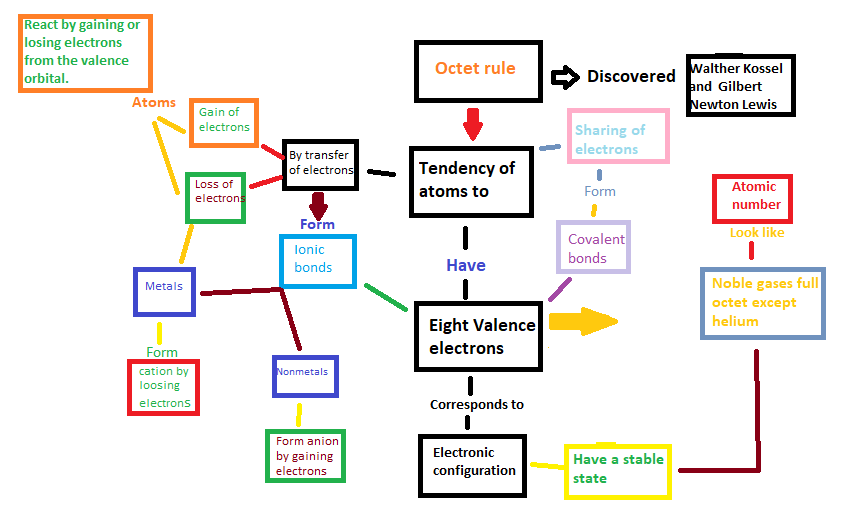
Transcribed Image Text:React by gaining or
losing electrons
from the valence
orbital.
Atoms
Metals
Form
cation by
loosing
electrons
Gain of
electrons
Loss of
electrons
By transfer
of electrons
Form
Ionic
bonds
Nonmetals
Form anion
by gaining
electrons
Octet rule
Tendency of
atoms to
Have
Eight Valence
electrons
Corresponds to
Electronic
configuration
Discovered
Sharing of
electrons
I
Form
Covalent
bonds
Have a stable
state
Walther Kossel
and Gilbert
Newton Lewis
Atomic
number
Look like
I
Noble gases full
octet except
helium
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning
