Critical point- 72.9 Solid Liquid 67 BO 5.11 Triple point Vapour 1 A Temperature, TIK Figure 4A.8 The experimental phase diagram for carbon dioxide; note the break in the vertical scale. As the triple point lies at pressures well above atmospheric, liquid carbon dioxide does not exist under normal conditions; a pressure of at least 5.11 atm must be applied for liquid to be formed. The path ABCD is discussed in Brief illustration 4A.6. Pressure, platm 216.8 298.15 304.2
Critical point- 72.9 Solid Liquid 67 BO 5.11 Triple point Vapour 1 A Temperature, TIK Figure 4A.8 The experimental phase diagram for carbon dioxide; note the break in the vertical scale. As the triple point lies at pressures well above atmospheric, liquid carbon dioxide does not exist under normal conditions; a pressure of at least 5.11 atm must be applied for liquid to be formed. The path ABCD is discussed in Brief illustration 4A.6. Pressure, platm 216.8 298.15 304.2
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter6: Equilibria In Single-component Systems
Section: Chapter Questions
Problem 6.25E: 6.25. Phosphorus exists as several allotropes that have varying properties. The enthalpy of...
Related questions
Question
Refer to Fig. 4A.8. Which phase or phases would you expect to be present for a sample of CO2 at: (i) 200 K and 2.5 atm; (ii) 300 K and 4 atm; (iii) 310 K and 50 atm?
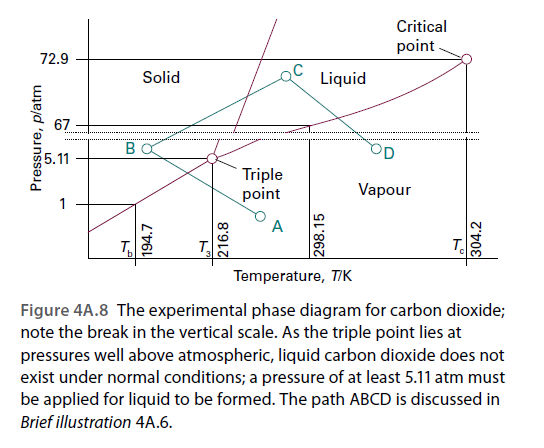
Transcribed Image Text:Critical
point-
72.9
Solid
Liquid
67
BO
5.11
Triple
point
Vapour
1
A
Temperature, TIK
Figure 4A.8 The experimental phase diagram for carbon dioxide;
note the break in the vertical scale. As the triple point lies at
pressures well above atmospheric, liquid carbon dioxide does not
exist under normal conditions; a pressure of at least 5.11 atm must
be applied for liquid to be formed. The path ABCD is discussed in
Brief illustration 4A.6.
Pressure, platm
216.8
298.15
304.2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,