d. In another experiment, the student titrated 50.0 mL of 0.100 M HC2H3O2 with 0.100 M NaOH. Calculate the pH of the solution at the equivalence point.
d. In another experiment, the student titrated 50.0 mL of 0.100 M HC2H3O2 with 0.100 M NaOH. Calculate the pH of the solution at the equivalence point.
Chapter19: Applications Of Standard Electrode Potentials
Section: Chapter Questions
Problem 19.13QAP
Related questions
Question
d. In another experiment, the student titrated 50.0 mL of 0.100 M HC2H3O2 with 0.100 M NaOH.
Calculate the pH of the solution at the equivalence point.
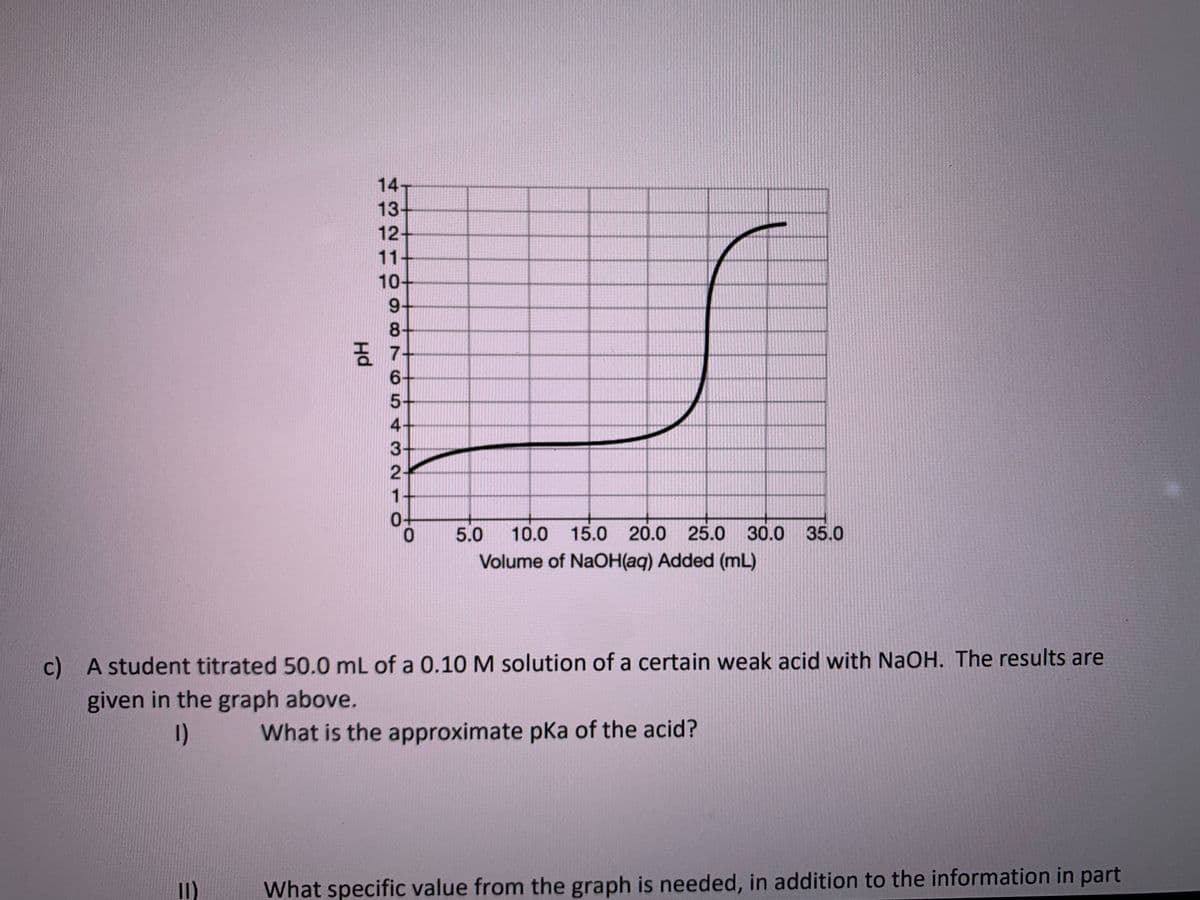
Transcribed Image Text:14-
13-
12+
11
10-
9+
8.
5-
4
3
1
5.0
10.0 15.0 20.0 25.0 30.0 35.0
Volume of NaOH(aq) Added (mL)
c) A student titrated 50.0 mL of a 0.10 M solution of a certain weak acid with NaOH. The results are
given in the graph above.
I)
What is the approximate pKa of the acid?
I)
What specific value from the graph is needed, in addition to the information in part
Expert Solution
Step 1
The pH of the solution is the power of protons in the solution. While pOH is related to the basicity of the solution. pOH is calculated by subtracting the pH value from 14. The pH value for acids is in the range 1 to 7, for the base, it is 7 to 14, and 7 is the pH for a neutral solution.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning