Darlana Finding half life and rate constant from a graph of concentratio... A chemical engineer is studying the rate of this reaction 2NH3 g)N2 (g)+3H2 (g) He fills a reaction vessel with NH2 and measures its concentration as the reaction proceeds. Here's a graph of his data: dlo (seconds) ()[HN lII U KINETICS AND EQUILIBRIUM Dariana Finding half life and rate constant from a graph of concentratio... Use this graph to answer the following questions: What is the half life of the reaction? to Round your answer to 2 significant digits. Suppose the rate of the reaction is known to be first order in NH2. Calculate the value of the rate constant k. X k = Round your answer to 2 significant digits. Also be sure you include the correct unit symbol Ar Predict the concentration of NH2 in the engineer's reaction vessel after 6.0 seconds have passed NH, M Assume no other reaction is important, and continue to assume the rate is first order in NH3. Round your answer to 2 significant digits.
Darlana Finding half life and rate constant from a graph of concentratio... A chemical engineer is studying the rate of this reaction 2NH3 g)N2 (g)+3H2 (g) He fills a reaction vessel with NH2 and measures its concentration as the reaction proceeds. Here's a graph of his data: dlo (seconds) ()[HN lII U KINETICS AND EQUILIBRIUM Dariana Finding half life and rate constant from a graph of concentratio... Use this graph to answer the following questions: What is the half life of the reaction? to Round your answer to 2 significant digits. Suppose the rate of the reaction is known to be first order in NH2. Calculate the value of the rate constant k. X k = Round your answer to 2 significant digits. Also be sure you include the correct unit symbol Ar Predict the concentration of NH2 in the engineer's reaction vessel after 6.0 seconds have passed NH, M Assume no other reaction is important, and continue to assume the rate is first order in NH3. Round your answer to 2 significant digits.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.48PAE: 11.48 The following data were collected for the decomposition of NT),-: Time, f (min) [N2Os] (mol...
Related questions
Question
Transcribed Image Text:Darlana
Finding half life and rate constant from a graph of concentratio...
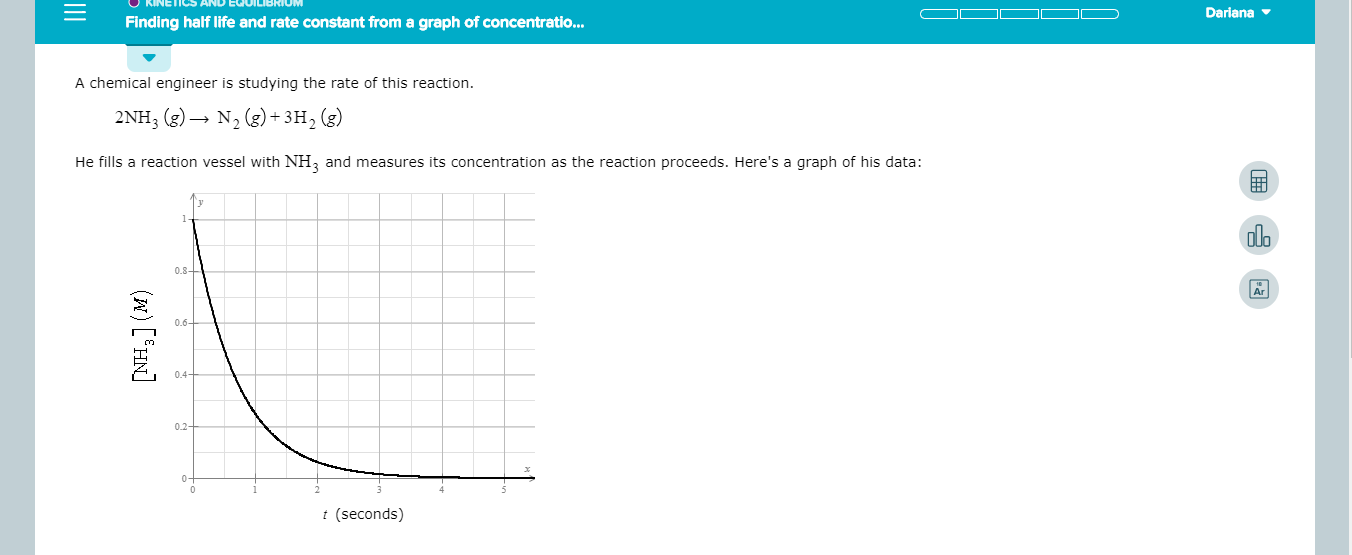
A chemical engineer is studying the rate of this reaction
2NH3 g)N2 (g)+3H2 (g)
He fills a reaction vessel with NH2 and measures its concentration as the reaction proceeds. Here's a graph of his data:
dlo
(seconds)
()[HN
lII
Transcribed Image Text:U KINETICS AND EQUILIBRIUM
Dariana
Finding half life and rate constant from a graph of concentratio...
Use this graph to answer the following questions:
What is the half life of the reaction?
to
Round your answer to 2 significant digits.
Suppose the rate of the reaction is known to be first order in
NH2. Calculate the value of the rate constant k.
X
k =
Round your answer to 2 significant digits. Also be sure you
include the correct unit symbol
Ar
Predict the concentration of NH2 in the engineer's reaction
vessel after 6.0 seconds have passed
NH, M
Assume no other reaction is important, and continue to
assume the rate is first order in NH3.
Round your answer to 2 significant digits.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning