Density is an intensive physical property that relates the mass of an object to its volume. Density, which is simply the mass of an object divided by its volume, is expressed in the SI derived unit g/mL for a liquid or g/cm³ for a solid. Most substances Part A expand or contract when heated or cooled, so the density values for a substance are temperature dependent. A particular brand of gasoline has a density of 0.737 g/mL at 25 °C. How many grams of this gasoline would fill a 16.0 gal tank (1 US gal = 3.78 L)? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) ? Value Units Part B A block of metal has a width of 3.2 cm, a length of 17.1 cm, and height of 3.5 cm . Its mass is 1.3 kg. Calculate the density of the metal. Express your answer to two significant figures and include the appropriate units. • View Available Hint(s) HẢ ? Value Units
Density is an intensive physical property that relates the mass of an object to its volume. Density, which is simply the mass of an object divided by its volume, is expressed in the SI derived unit g/mL for a liquid or g/cm³ for a solid. Most substances Part A expand or contract when heated or cooled, so the density values for a substance are temperature dependent. A particular brand of gasoline has a density of 0.737 g/mL at 25 °C. How many grams of this gasoline would fill a 16.0 gal tank (1 US gal = 3.78 L)? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) ? Value Units Part B A block of metal has a width of 3.2 cm, a length of 17.1 cm, and height of 3.5 cm . Its mass is 1.3 kg. Calculate the density of the metal. Express your answer to two significant figures and include the appropriate units. • View Available Hint(s) HẢ ? Value Units
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter3: Matter
Section: Chapter Questions
Problem 5CR
Related questions
Question
Please answer question 14 parts A and B
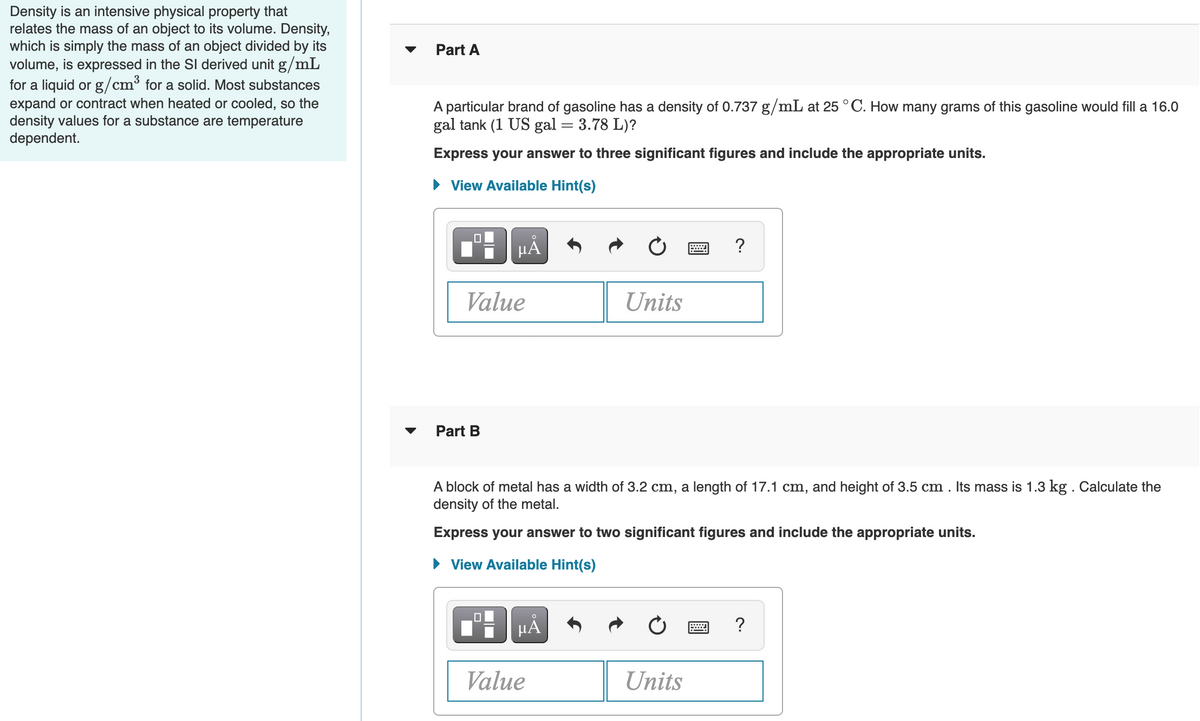
Transcribed Image Text:Density is an intensive physical property that
relates the mass of an object to its volume. Density,
which is simply the mass of an object divided by its
volume, is expressed in the SI derived unit g/mL
for a liquid or g/cm³ for a solid. Most substances
expand or contract when heated or cooled, so the
density values for a substance are temperature
dependent.
Part A
A particular brand of gasoline has a density of 0.737 g/mL at 25 ° C. How many grams of this gasoline would fill a 16.0
gal tank (1 US gal = 3.78 L)?
Express your answer to three significant figures and include the appropriate units.
View Available Hint(s)
HẢ
?
Value
Units
Part B
A block of metal has a width of 3.2 cm, a length of 17.1 cm, and height of 3.5 cm . Its mass is 1.3 kg . Calculate the
density of the metal.
Express your answer to two significant figures and include the appropriate units.
View Available Hint(s)
HẢ
?
Value
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER
