Describe any visual differences between the hydrated sample and the dried, anhydrous form. How would the following errors affect the empirical formula for the compound? a. The student ran out of time and did not do the second heating. Explain how this error will affect the calculation for the number of moles of water in the hydrate? Will the final answer be artificially high or low? How do you know? b. The student recorded the mass of the cup + sample incorrectly and started with 2.2 g of hydrated compound but used 2.0 g in the calculations. Explain how this error will affect the calculation for the number of moles of water in the hydrate? Will the final answer be artificially high or low? How do you know?
Describe any visual differences between the hydrated sample and the dried, anhydrous form. How would the following errors affect the empirical formula for the compound? a. The student ran out of time and did not do the second heating. Explain how this error will affect the calculation for the number of moles of water in the hydrate? Will the final answer be artificially high or low? How do you know? b. The student recorded the mass of the cup + sample incorrectly and started with 2.2 g of hydrated compound but used 2.0 g in the calculations. Explain how this error will affect the calculation for the number of moles of water in the hydrate? Will the final answer be artificially high or low? How do you know?
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.16QAP
Related questions
Question
- Describe any visual differences between the hydrated sample and the dried, anhydrous form.
- How would the following errors affect the empirical formula for the compound? a. The student ran out of time and did not do the second heating. Explain how this error will affect the calculation for the number of moles of water in the hydrate? Will the final answer be artificially high or low? How do you know? b. The student recorded the mass of the cup + sample incorrectly and started with 2.2 g of hydrated compound but used 2.0 g in the calculations. Explain how this error will affect the calculation for the number of moles of water in the hydrate? Will the final answer be artificially high or low? How do you know?
Transcribed Image Text:Important
Karına
3. Write the
to answer th
Experiment 2
EE Data Table 2
Photo 2
Photo 3
Photo 4
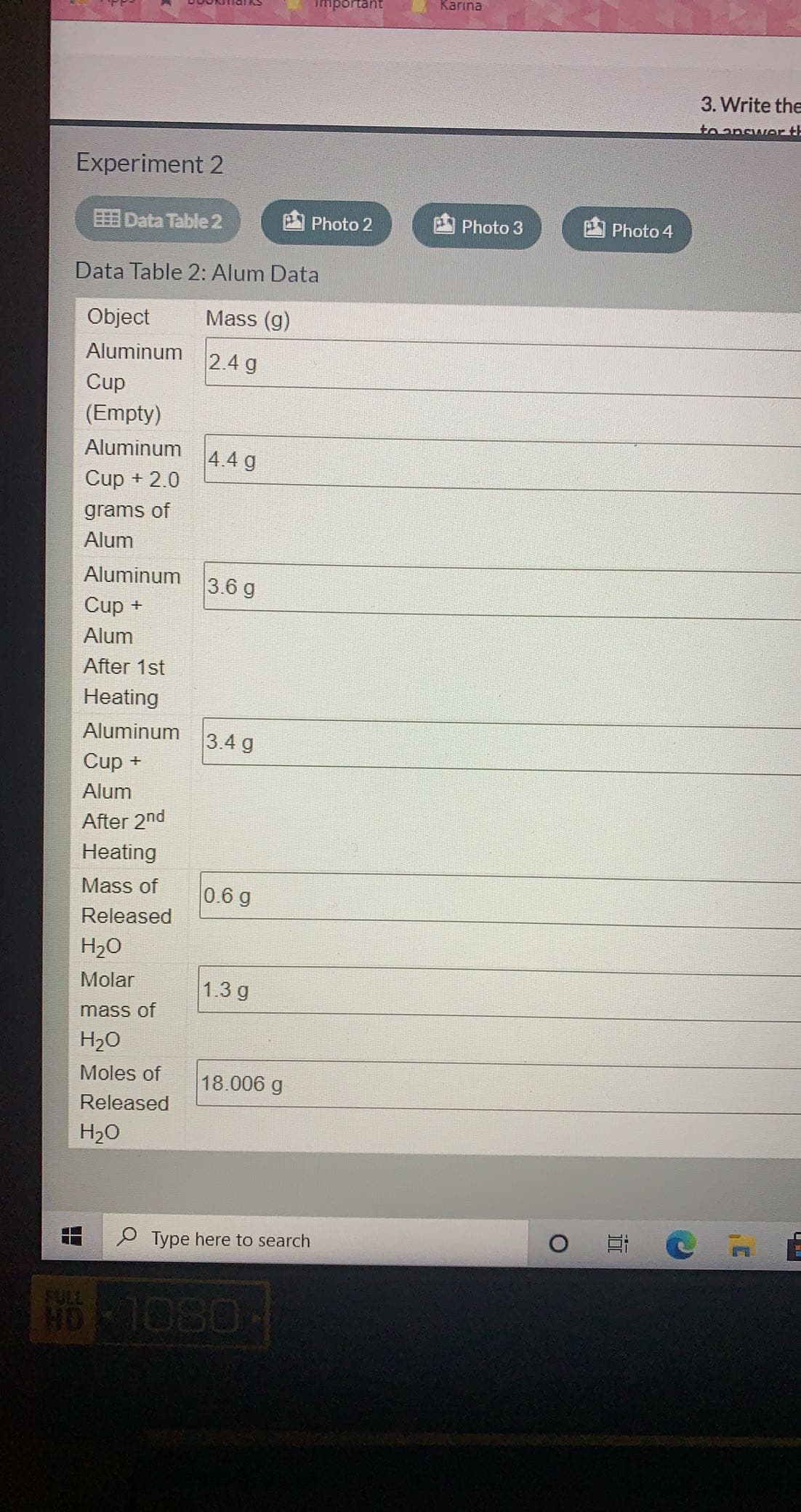
Data Table 2: Alum Data
Object
Mass (g)
Aluminum
2.4 g
Cup
(Empty)
Aluminum
4.4 g
Cup + 2.0
grams of
Alum
Aluminum
3.6 g
Cup +
Alum
After 1st
Heating
Aluminum
3.4 g
Cup +
Alum
After 2nd
Heating
Mass of
0.6 g
Released
H2O
Molar
1.3 g
mass of
H2O
Moles of
18.006 g
Released
H20
P Type here to search
HD 1080
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



