Determine the percent abundance for J-67 and J-68, given the following data: The average atomic mass for J is 65.96 amu. Atomic % Isotope weight abundance J-64 63.9296 6.744 J-66 65.9449 90.654 |J-67 66.9154 ?? J-68 67.9113 ??
Determine the percent abundance for J-67 and J-68, given the following data: The average atomic mass for J is 65.96 amu. Atomic % Isotope weight abundance J-64 63.9296 6.744 J-66 65.9449 90.654 |J-67 66.9154 ?? J-68 67.9113 ??
Chapter1: Excel Basics
Section: Chapter Questions
Problem 4P
Related questions
Question
Solve:
The
65.96 = 63.9296 ×6.744 + 65.9449 × 90.654 + 67.9113 × M + 66.9154 × (100− (M+6.744+90.654))/100
65.96 = 431.1412 + 5978.169 + 67.9113 M + 66.9154 × (2.602−M)/100
6596 =
MM=
The abundance of J−68 is
The abundance of J−67 is
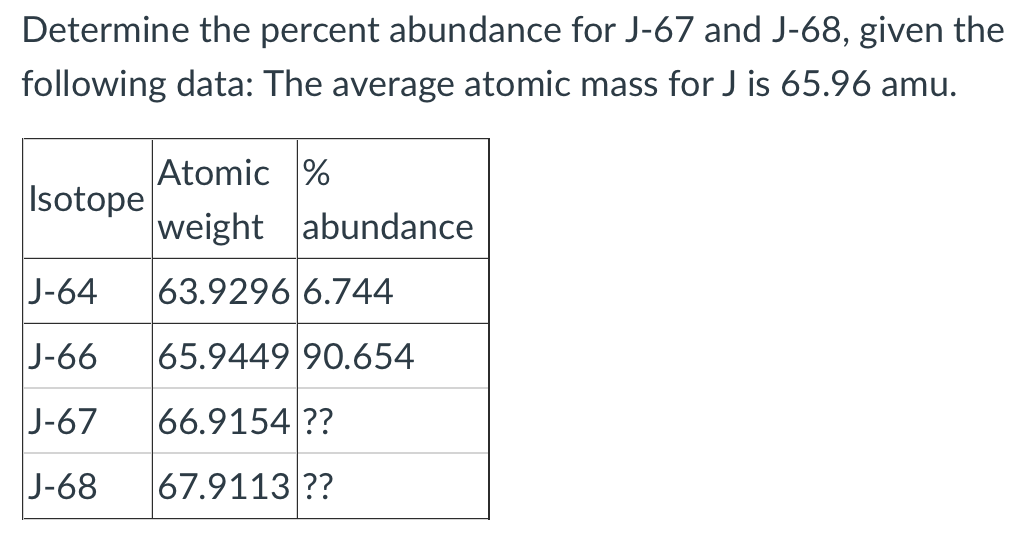
Transcribed Image Text:Determine the percent abundance for J-67 and J-68, given the
following data: The average atomic mass for J is 65.96 amu.
Atomic %
Isotope
weight abundance
J-64
63.9296 6.744
J-66
65.9449 90.654
|J-67
66.9154 ??
J-68
67.9113 ??
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning



Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning