Dichlorodifluoromethane, once widely used as a refrigerant, can be prepared by the reactions shown How many moles of Cl2 must be consumed in the first reaction to produce 2.25 kg CCI2F2 in the second? Assume that all the CC4 produced in the first reaction is consumed in the second. cC4 +HCl (not balanced) CC2F2 +HCl (not balanced) CH4C2 CC4HF
Dichlorodifluoromethane, once widely used as a refrigerant, can be prepared by the reactions shown How many moles of Cl2 must be consumed in the first reaction to produce 2.25 kg CCI2F2 in the second? Assume that all the CC4 produced in the first reaction is consumed in the second. cC4 +HCl (not balanced) CC2F2 +HCl (not balanced) CH4C2 CC4HF
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 30A
Related questions
Question
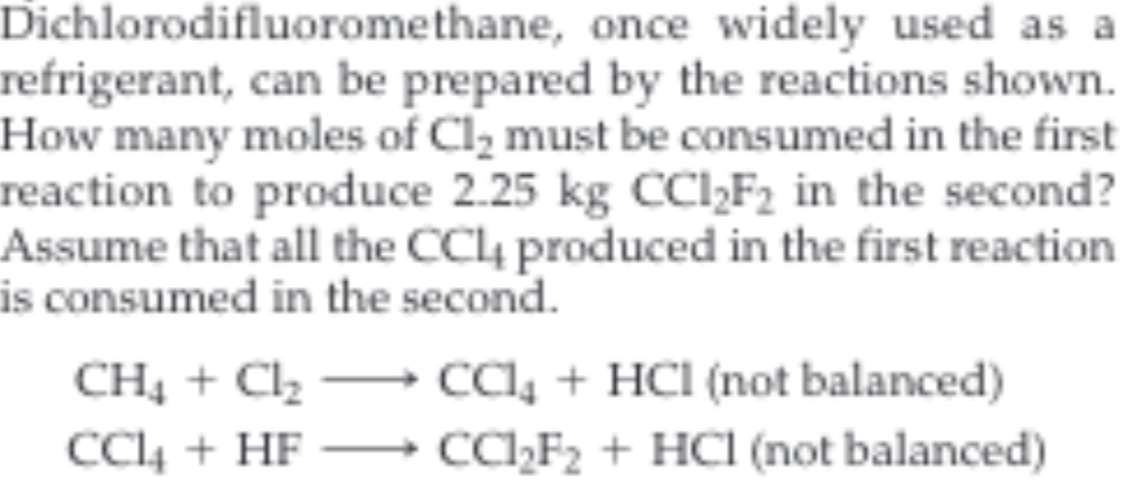
Transcribed Image Text:Dichlorodifluoromethane, once widely used as a
refrigerant, can be prepared by the reactions shown
How many moles of Cl2 must be consumed in the first
reaction to produce 2.25 kg CCI2F2 in the second?
Assume that all the CC4 produced in the first reaction
is consumed in the second.
cC4 +HCl (not balanced)
CC2F2 +HCl (not balanced)
CH4C2
CC4HF
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning