:DICTIONS 27. Use the process you learned to predict the formula for the salt that will form when rubidium (Rb) bonds with fluorine (F). 8. 28. Predict the formula for the salt that will form when rubidium (Rb) bonds with nitrogen (N).
:DICTIONS 27. Use the process you learned to predict the formula for the salt that will form when rubidium (Rb) bonds with fluorine (F). 8. 28. Predict the formula for the salt that will form when rubidium (Rb) bonds with nitrogen (N).
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.42PAE: Amoxicillin is an antibiotic packaged as a powder. When it is used to treat babies and small...
Related questions
Question
Please question 27 and 28
Transcribed Image Text:2:32 pm On time
8 /9 >
195%
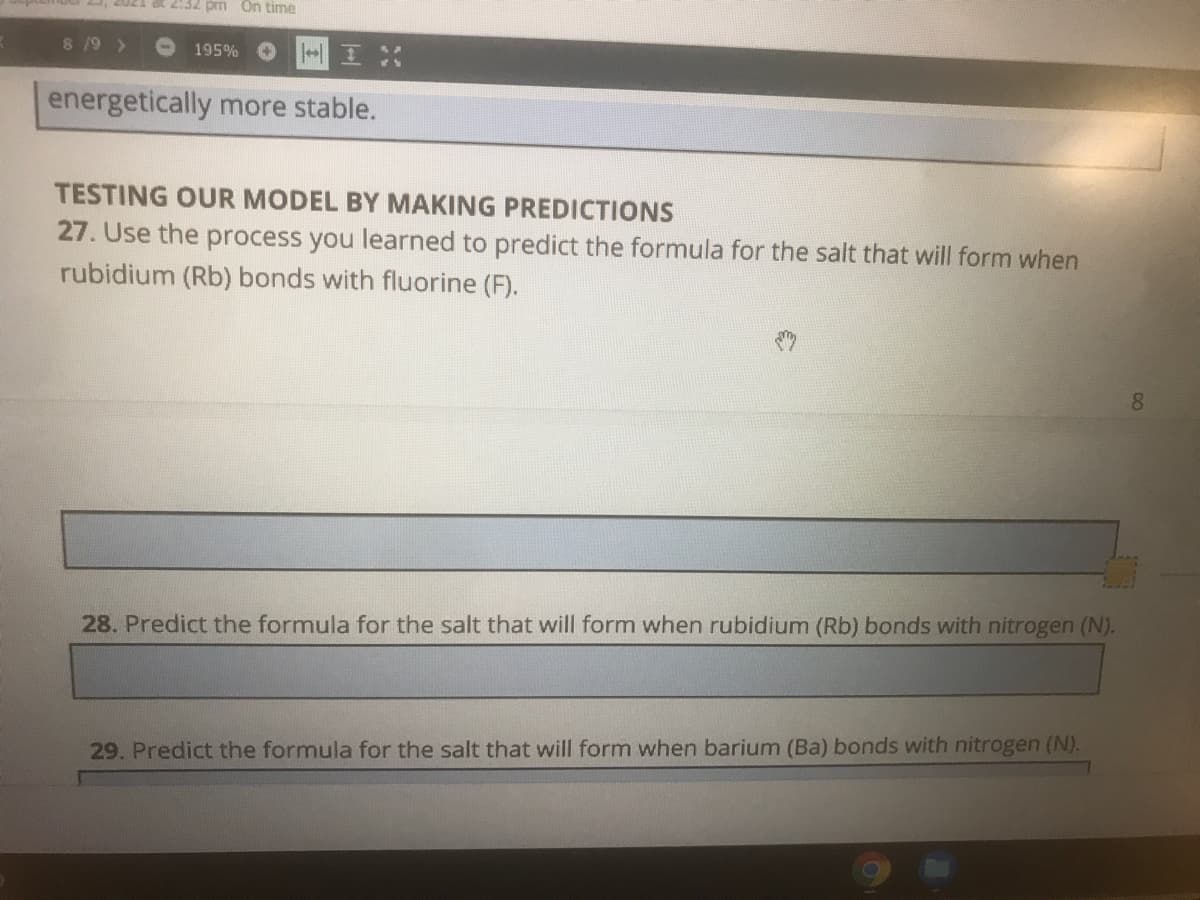
energetically more stable.
TESTING OUR MODEL BY MAKING PREDICTIONS
27. Use the process you learned to predict the formula for the salt that will form when
rubidium (Rb) bonds with fluorine (F).
28. Predict the formula for the salt that will form when rubidium (Rb) bonds with nitrogen (N).
29. Predict the formula for the salt that will form when barium (Ba) bonds with nitrogen (N).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning