Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter14: Mass Spectrometry
Section: Chapter Questions
Problem 14.37P
Related questions
Question
( do 22 with explanation
Transcribed Image Text:4.20. SOLI
No par
1.
alkane
becaus
(no M')
30 4
50
60
70
80
90
100
110
120
130 140
m/z
Fig. 4.14
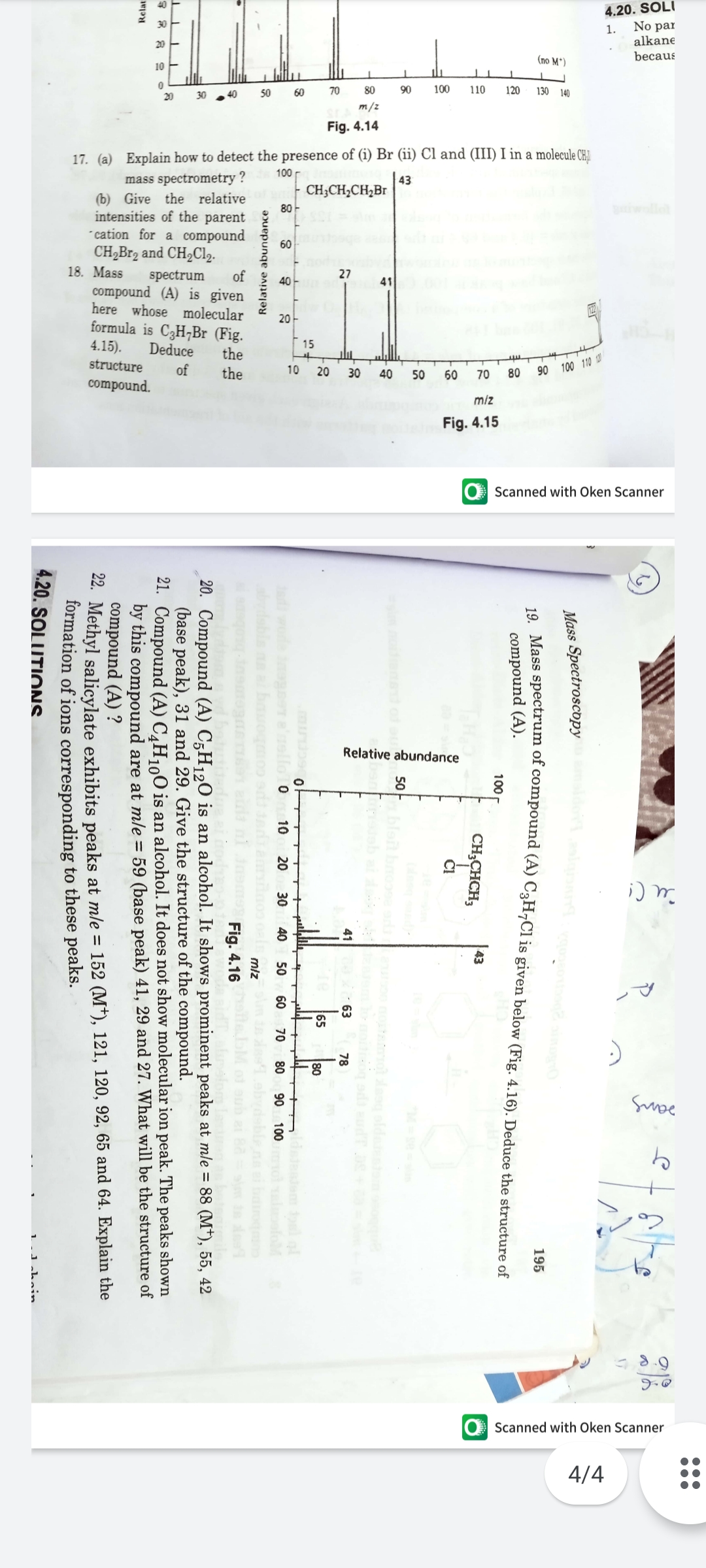
17. (a) Explain how to detect the presence of (i) Br (ii) Cl and (III) I in a molecule CH
mass spectrometry
| 43
| CH;CH,CH,Br
80
(b) Give the relative
60
spectrum
of
40
41
20-
Deduce
15
the
of
10 20
80 90 100 110
the
30
40
50
60
70
miz
Fig. 4.15
O Scanned with Oken Scanner
Relative abundance
レマ
డి
O Scanned with Oken Scanner
4/4
Mass Spectroscopy
195
compound (A).
100
43
CH;CHCH;
Č!
50
Tat bro
eb a
41
63
78
65
80
de ager e'ello 0
bdsbis n
10
20
30
40
50
60
70
80
90
100
ofoM
Relative abundance
miz
Fig. 4.16
20. Compound (A) C;H120 is an alcohol. It shows prominent peaks at mle = 88 (M*), 55, 42
(base peak), 31 and 29. Give the structure of the compound.
21. Compound (A) C¸H1,O is an alcohol. It does not show molecular ion peak. The peaks shown
by this compound are at mle = 59 (base peak) 41, 29 and 27. What will be the structure of
compound (A) ?
22. Methyl salicylate exhibits peaks at mle = 152 (M*), 121, 120, 92, 65 and 64. Explain the
formation of ions corresponding to these peaks.
4.20. SOLUTIONS.
Relat
1.Jahoin
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning