the reactants and products. Name the final ester product. *draw your structures so the functional groups "line up" and circle the water that is being removed a. names C. structural formulas b. names structural formulas names Structural formulas d. names Structural formulas Reactants butanoic acid + ethanol → butanoic acid + pentan-1-ol ethanoic acid + propan-2-ol ethanoic acid + octan-1-ol Product
the reactants and products. Name the final ester product. *draw your structures so the functional groups "line up" and circle the water that is being removed a. names C. structural formulas b. names structural formulas names Structural formulas d. names Structural formulas Reactants butanoic acid + ethanol → butanoic acid + pentan-1-ol ethanoic acid + propan-2-ol ethanoic acid + octan-1-ol Product
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter18: Carboxylic Acids
Section: Chapter Questions
Problem 18.54P
Related questions
Question
Transcribed Image Text:Esters
Many naturally occurring esters are responsible for the pleasant, characteristic smells of various fruits. As a result,
synthetic esters are commonly made for use in perfumes and artificial flavours.
To produce a synthetic ester with a specific smell, chemists research what ester will give them the specific smell they are
interested in and then they work "backwards" to design a chemical reaction pathway to produce the desired ester using
commonly available chemicals.
Esters are commonly produced by reacting a carboxylic acid with an alcohol, if the required carboxylic acid and alcohol
are not commonly available, chemists have to use a multi-step chemical reaction pathway to first produce the carboxylic
acid and/or alcohol, and then produce the desired ester.
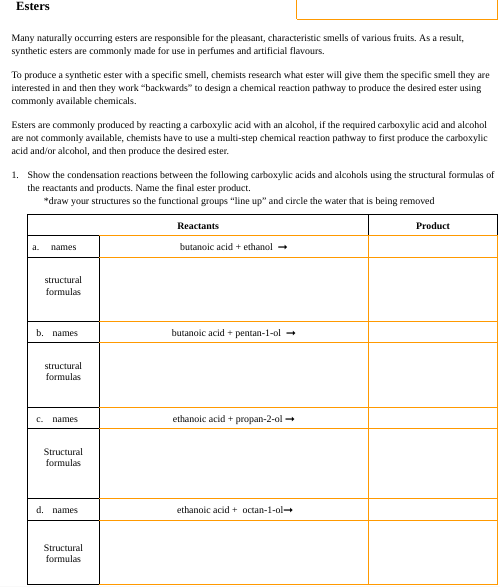
1. Show the condensation reactions between the following carboxylic acids and alcohols using the structural formulas of
the reactants and products. Name the final ester product.
*draw your structures so the functional groups "line up" and circle the water that is being removed
a.
names
C.
structural
formulas
b. names.
structural
formulas
names
Structural
formulas
d. names
Structural
formulas
Reactants
butanoic acid + ethanol →
butanoic acid + pentan-1-ol →
ethanoic acid + propan-2-ol →
ethanoic acid + octan-1-ol
Product
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
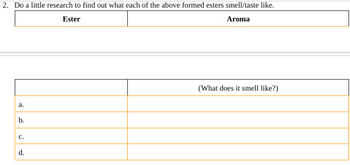
Transcribed Image Text:2. Do a little research to find out what each of the above formed esters smell/taste like.
Ester
Aroma
a.
b.
C.
d.
(What does it smell like?)
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning