Dr. Alghe has just performed a restriction digest on DNA she extracted from her samples. She must now run her DNA out on a gel to separate the fragments based on size. To do this she must prepare a 1.8% agarose gel, where the percentage is determined as mass/vol. How many grams of agarose must she add to 125 mL of buffer in order to arrive at the correct percentage? (answer to 2 decimals) **Assume the dissolution of agarose will not alter the final volume of the solution** Polymerase Chain Reaction (PCR) is an essential technique for the study of DNA. PCR uses a DNA polymerase enzyme from Thermophilus aquaticus to amplify DNA extracted from any sample. In order for the reaction to work, researchers must transfer microliters of sample to the reaction vessel for PCR. If a researcher has a DNA sample with a concentration of 0.029 micrograms per microliter, how many microliters of DNA must be transfered to conduct a 50 microliter reaction if the final concentration should be 4.6 nanograms per microliter? Report your answer to two decimal places using normal decimal notation (e.g. 0.5, not 5.0e-1)
Dr. Alghe has just performed a restriction digest on DNA she extracted from her samples. She must now run her DNA out on a gel to separate the fragments based on size. To do this she must prepare a 1.8% agarose gel, where the percentage is determined as mass/vol. How many grams of agarose must she add to 125 mL of buffer in order to arrive at the correct percentage? (answer to 2 decimals) **Assume the dissolution of agarose will not alter the final volume of the solution** Polymerase Chain Reaction (PCR) is an essential technique for the study of DNA. PCR uses a DNA polymerase enzyme from Thermophilus aquaticus to amplify DNA extracted from any sample. In order for the reaction to work, researchers must transfer microliters of sample to the reaction vessel for PCR. If a researcher has a DNA sample with a concentration of 0.029 micrograms per microliter, how many microliters of DNA must be transfered to conduct a 50 microliter reaction if the final concentration should be 4.6 nanograms per microliter? Report your answer to two decimal places using normal decimal notation (e.g. 0.5, not 5.0e-1)
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter31: Immunochemistry
Section: Chapter Questions
Problem 31.20P: Chemical Connections 20D states that the antigen in the red blood cells of a person with B-type...
Related questions
Question
please highlight the correct answer
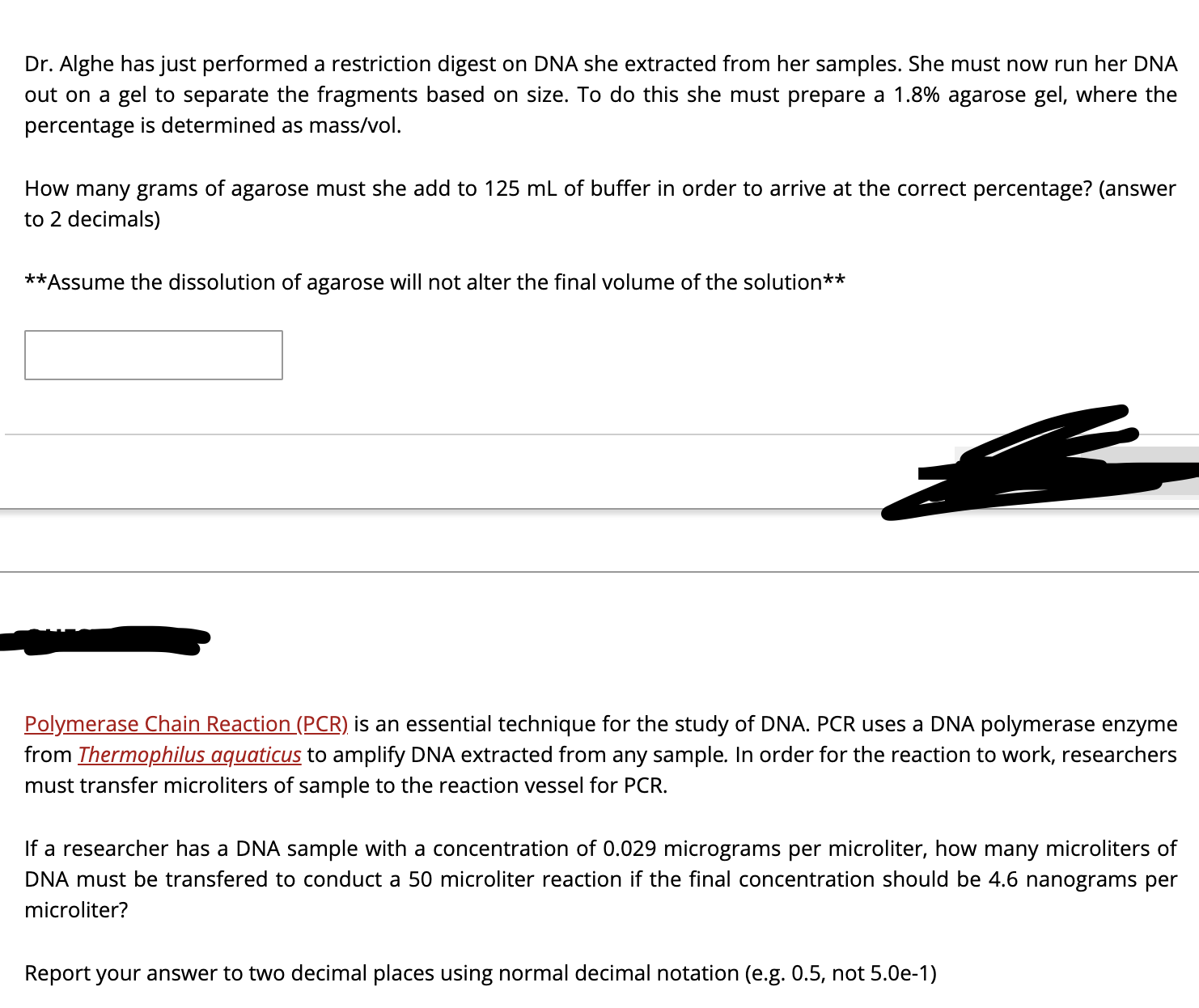
Transcribed Image Text:Dr. Alghe has just performed a restriction digest on DNA she extracted from her samples. She must now run her DNA
out on a gel to separate the fragments based on size. To do this she must prepare a 1.8% agarose gel, where the
percentage is determined as mass/vol.
How many grams of agarose must she add to 125 mL of buffer in order to arrive at the correct percentage? (answer
to 2 decimals)
**Assume the dissolution of agarose will not alter the final volume of the solution**
Polymerase Chain Reaction (PCR) is an essential technique for the study of DNA. PCR uses a DNA polymerase enzyme
from Thermophilus aquaticus to amplify DNA extracted from any sample. In order for the reaction to work, researchers
must transfer microliters of sample to the reaction vessel for PCR.
If a researcher has a DNA sample with a concentration of 0.029 micrograms per microliter, how many microliters of
DNA must be transfered to conduct a 50 microliter reaction if the final concentration should be 4.6 nanograms per
microliter?
Report your answer to two decimal places using normal decimal notation (e.g. 0.5, not 5.0e-1)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning