E MasteringChemistry: HW 01b C Get Homework Help With Che b My Questions | bartleby > MyLab and Mastering Home - Students x X A session.masteringchemistry.com/myct/itemView?offset=next&attemptNo=1&assignmentProblemlD=139293464 E Apps W California Fool's Go... CLA Purdue OWL: APA F.. O Christmas Window... C Bianca 4-piece Fabr.. O ANGELINO o How do I format m. O Choosing Resource. New Tab Dashboard >>
E MasteringChemistry: HW 01b C Get Homework Help With Che b My Questions | bartleby > MyLab and Mastering Home - Students x X A session.masteringchemistry.com/myct/itemView?offset=next&attemptNo=1&assignmentProblemlD=139293464 E Apps W California Fool's Go... CLA Purdue OWL: APA F.. O Christmas Window... C Bianca 4-piece Fabr.. O ANGELINO o How do I format m. O Choosing Resource. New Tab Dashboard >>
Chapter2: Basic Statistical Analysis With Excel
Section: Chapter Questions
Problem 12P
Related questions
Question
Transcribed Image Text:E MasteringChemistry: HW 01b
C Get Homework Help With Che
b My Questions | bartleby
> MyLab and Mastering
Home - Students
x
X
A session.masteringchemistry.com/myct/itemView?offset=next&attemptNo=1&assignmentProblemlD=139293464
E Apps
W California Fool's Go...
CLA Purdue OWL: APA F..
O Christmas Window...
C Bianca 4-piece Fabr..
O ANGELINO
o How do I format m.
O Choosing Resource.
New Tab
Dashboard
>>
<HW 01b Mathematical Problem Solving and Conventions (Spring 2020) - Attempt 1
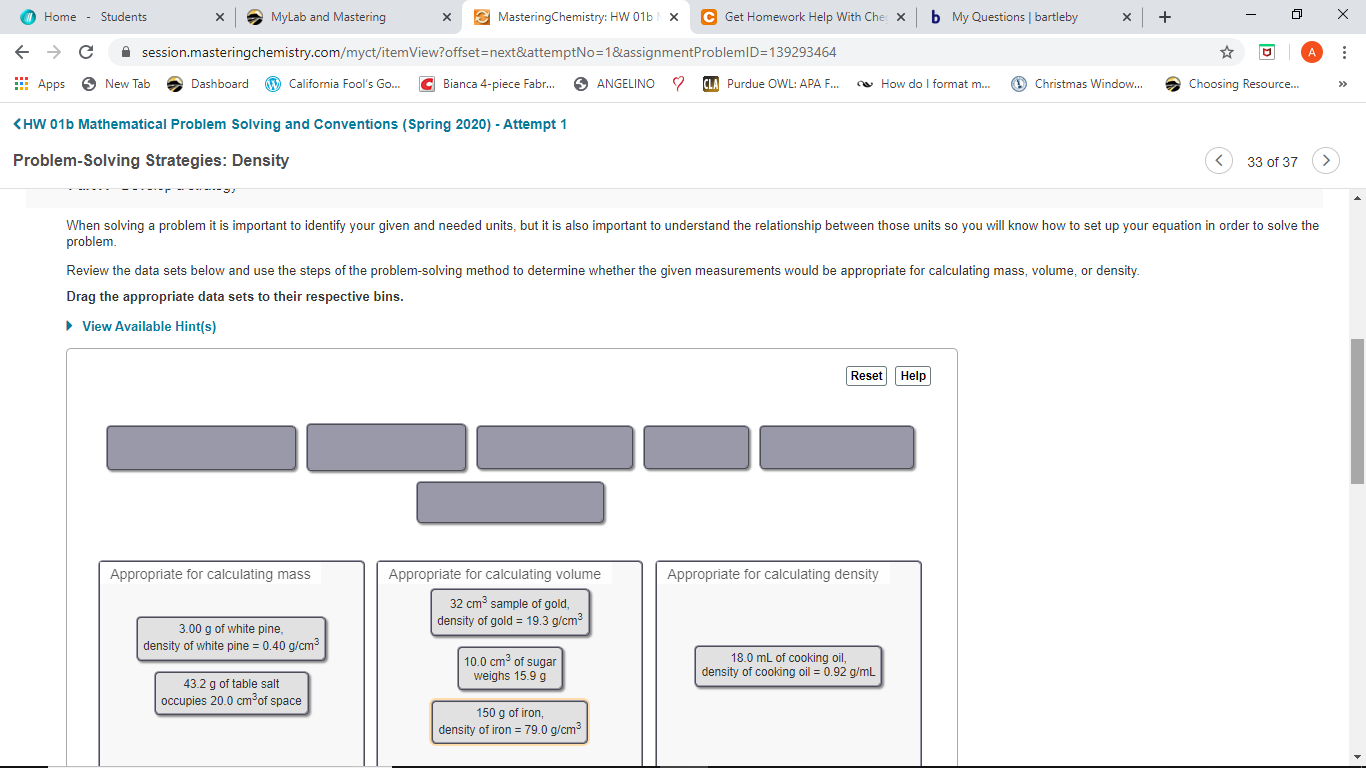
Problem-Solving Strategies: Density
33 of 37
When solving a problem it is important to identify your given and needed units, but it is also important to understand the relationship between those units so you will know how to set up your equation in order to solve the
problem.
Review the data sets below and use the steps of the problem-solving method to determine whether the given measurements would be appropriate for calculating mass, volume, or density.
Drag the appropriate data sets to their respective bins.
• View Available Hint(s)
Reset
Help
Appropriate for calculating mass
Appropriate for calculating volume
Appropriate for calculating density
32 cm3 sample of gold,
density of gold = 19.3 g/cm?
3.00 g of white pine,
density of white pine = 0.40 g/cm?
18.0 mL of cooking oil,
density of cooking oil = 0.92 g/mL
10.0 cm3 of sugar
weighs 15.9 g
43.2 g of table salt
occupies 20.0 cm of space
150 g of iron,
density of iron = 79.0 g/cm3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 6 images

Recommended textbooks for you

