e teaching and X com/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take&takeAssignmentSessionLocato Review Topics References Use the References to access important values if needed for this question. Identify whether each species functions as a Brønsted-Lowry acid or a Brønsted-Lowry base in this ne ionic equation. Clo HSO3 HCIO SO3 2 Bronsted-Lowry Bronsted-Lowry Brønsted-Lowry Brønsted-Lowry In this reaction: of ClO is The formula for the conjugate of HSO3 is The formula for the conjugate 4 more group attempts remaining Retry Entire Group Submit Answer Previous O verizon
e teaching and X com/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take&takeAssignmentSessionLocato Review Topics References Use the References to access important values if needed for this question. Identify whether each species functions as a Brønsted-Lowry acid or a Brønsted-Lowry base in this ne ionic equation. Clo HSO3 HCIO SO3 2 Bronsted-Lowry Bronsted-Lowry Brønsted-Lowry Brønsted-Lowry In this reaction: of ClO is The formula for the conjugate of HSO3 is The formula for the conjugate 4 more group attempts remaining Retry Entire Group Submit Answer Previous O verizon
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 16CR
Related questions
Question
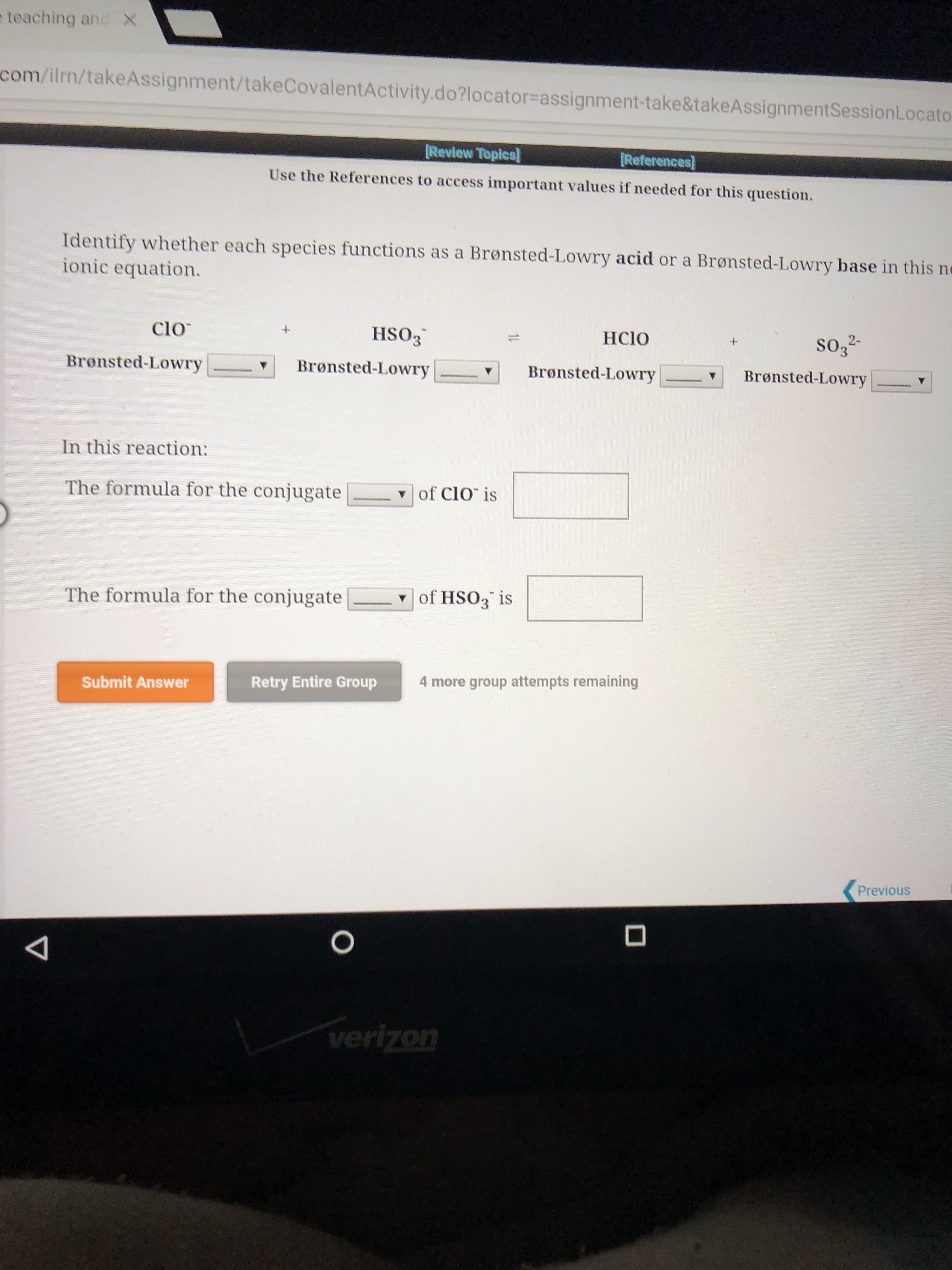
Transcribed Image Text:e teaching and
X
com/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take&takeAssignmentSessionLocato
Review Topics
References
Use the References to access important values if needed for this question.
Identify whether each species functions as a Brønsted-Lowry acid or a Brønsted-Lowry base in this ne
ionic equation.
Clo
HSO3
HCIO
SO3 2
Bronsted-Lowry
Bronsted-Lowry
Brønsted-Lowry
Brønsted-Lowry
In this reaction:
of ClO is
The formula for the conjugate
of HSO3 is
The formula for the conjugate
4 more group attempts remaining
Retry Entire Group
Submit Answer
Previous
O
verizon
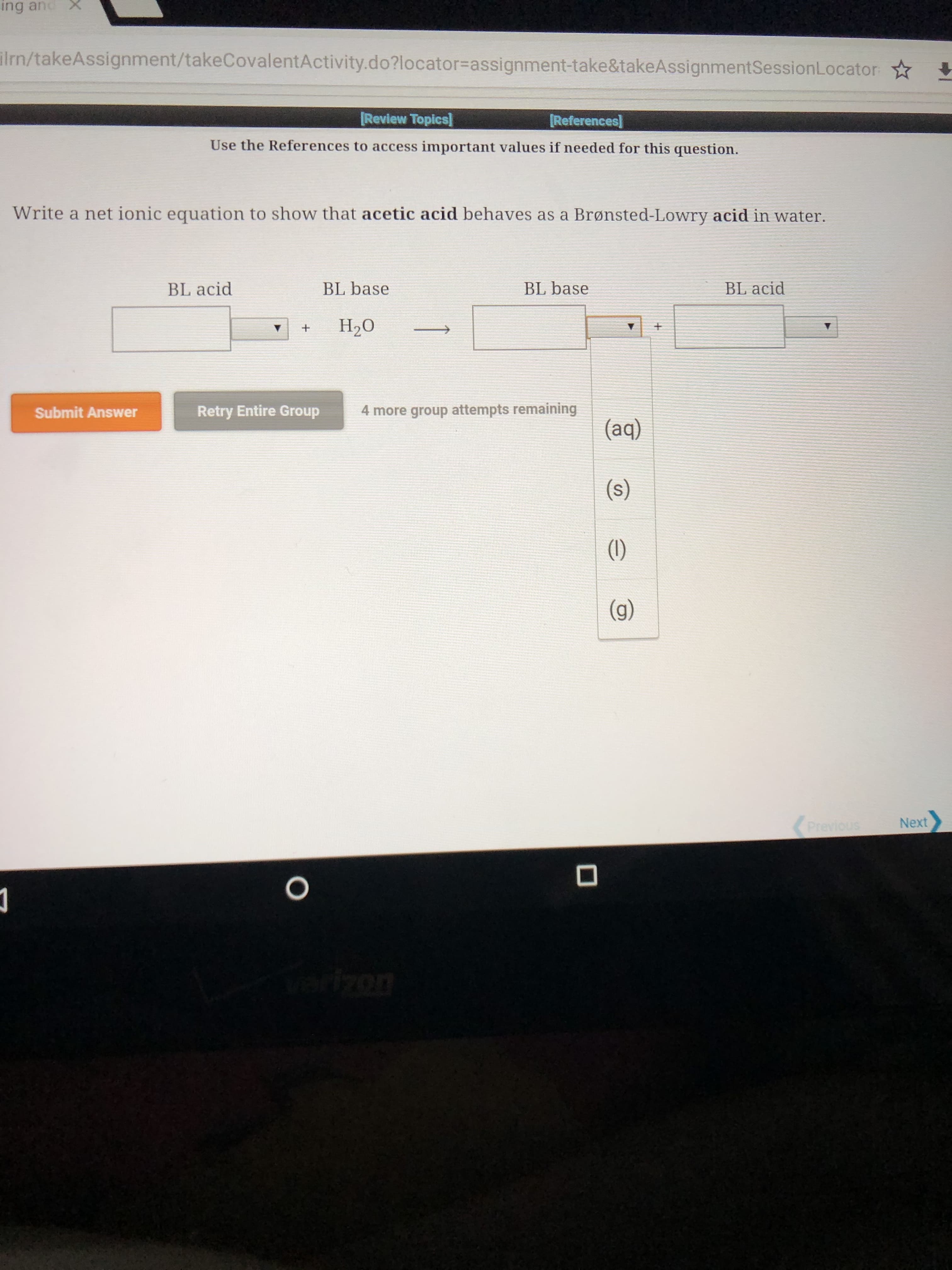
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning