e.com/course.html?courseld=15539865&HeplD=676f177415469ec3dc78ff3c8bf724d The Corinthian Ho... ersonalized Invit... Wholesale Glass... Shop | Origami Owl H 04 HW ing the Periodic Table to Write Electron Configurations Part C Write the complete electron configuration for gallium using the periodic table. Express your answer in complete form, in order of increasing orbital. For example, 1s2 View Available Hint(s) Electron configuration: You have already submitted this answer. Enter a new answer. No credit lost. Try again. Request Answer Previous Answers Submit Provide Feedback < Return to Assignment P Pearson Copyright O2019 Pearson Education Inc. All rights reserved. | Terms of U
e.com/course.html?courseld=15539865&HeplD=676f177415469ec3dc78ff3c8bf724d The Corinthian Ho... ersonalized Invit... Wholesale Glass... Shop | Origami Owl H 04 HW ing the Periodic Table to Write Electron Configurations Part C Write the complete electron configuration for gallium using the periodic table. Express your answer in complete form, in order of increasing orbital. For example, 1s2 View Available Hint(s) Electron configuration: You have already submitted this answer. Enter a new answer. No credit lost. Try again. Request Answer Previous Answers Submit Provide Feedback < Return to Assignment P Pearson Copyright O2019 Pearson Education Inc. All rights reserved. | Terms of U
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section: Chapter Questions
Problem 12ALQ
Related questions
Question
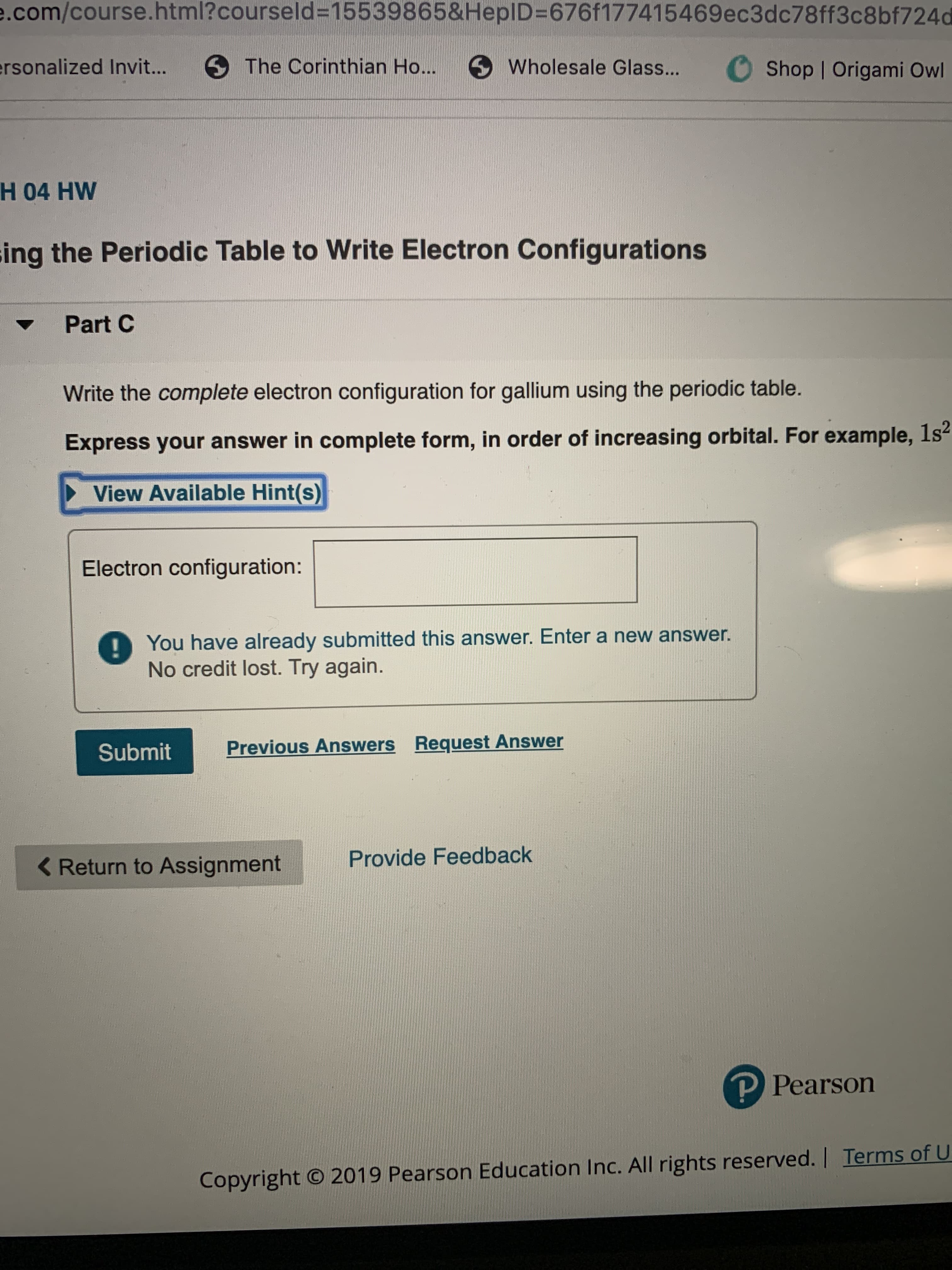
Transcribed Image Text:e.com/course.html?courseld=15539865&HeplD=676f177415469ec3dc78ff3c8bf724d
The Corinthian Ho...
ersonalized Invit...
Wholesale Glass...
Shop | Origami Owl
H 04 HW
ing the Periodic Table to Write Electron Configurations
Part C
Write the complete electron configuration for gallium using the periodic table.
Express your answer in complete form, in order of increasing orbital. For example, 1s2
View Available Hint(s)
Electron configuration:
You have already submitted this answer. Enter a new answer.
No credit lost. Try again.
Request Answer
Previous Answers
Submit
Provide Feedback
< Return to Assignment
P Pearson
Copyright O2019 Pearson Education Inc. All rights reserved. | Terms of U
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning