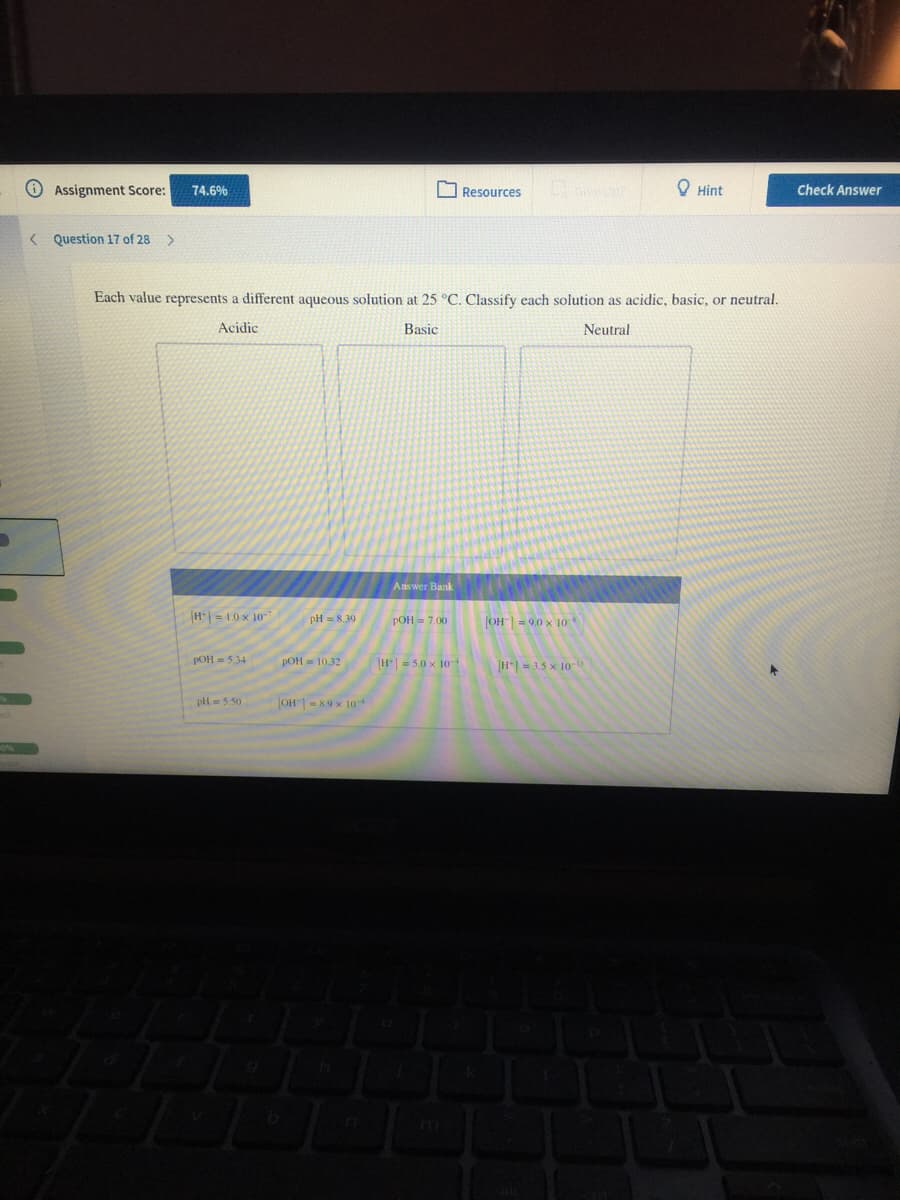
Each value represents a different aqueous solution at 25 °C. Classify each solution as acidic, basic, or neutral. Acidic Basic Neutral Answer Bank H= 10x 10 pH = 8.39 POH = 7.00 OH| = 9.0 x 10 H| = 5.0 x 10 POH = 534 POH= 10.32 H= 3.5 x 10 pli=550 JOH-89 x 10
Each value represents a different aqueous solution at 25 °C. Classify each solution as acidic, basic, or neutral. Acidic Basic Neutral Answer Bank H= 10x 10 pH = 8.39 POH = 7.00 OH| = 9.0 x 10 H| = 5.0 x 10 POH = 534 POH= 10.32 H= 3.5 x 10 pli=550 JOH-89 x 10
Chapter17: Introduction To Iv Therapy
Section: Chapter Questions
Problem 3.6P
Related questions
Question
Transcribed Image Text:Assignment Score:
74.6%
Resources
O Hint
Check Answer
< Question 17 of 28
Each value represents a different aqueous solution at 25 °C. Classify each solution as acidic, basic, or neutral.
Acidic
Basic
Neutral
Answer Bank
H|= 1.0 x 10-7
РОН 3 7.00
pH = 8,39
OH= 9.0 x 1o
POH = 5.34
POH = 10.32
H = 5.0 x 10
[H- 3.5 x 10-D
pH= 5.50
OH = 8.9 x 10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



